| 16698-35-4 |
| d-beta-Tocopherol |
| 148-03-8 |
| Cumotocopherol |
| beta Tocopherol |
| p-Xylotocopherol |
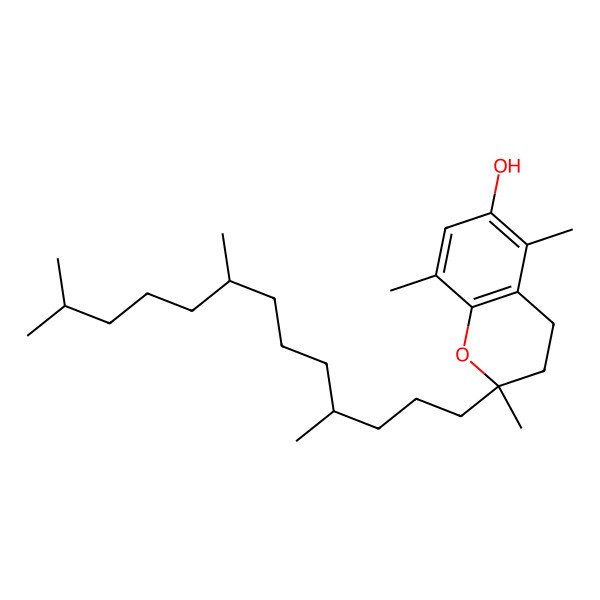
| (R)-2,5,8-Trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-ol |
| .beta.-Tocopherol |
| 5,8-Dimethyltocol |
| beta-D-Tocopherol |
| Neotocopherol |
| UNII-8K9365K9PX |
| (2R)-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol |
| 8K9365K9PX |
| UNII-9U6A490501 |
| EINECS 205-708-5 |
| EINECS 240-747-1 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, (2R)- |
| 9U6A490501 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)-, (2R)- |
| (+)-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol |
| DL-beta-Tocopherol |
| rel-(R)-2,5,8-Trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-ol |
| 3,4-Dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| (2R)-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol |
| (2R)-3,4-dihydro-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol |
| [2R[2R*(4R*,8R*)]]-3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)-, (2R)-rel- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, (2R)-rel- |
| C28H48O2 |
| (2R)-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)chroman-6-ol |
| beta -Tocopherol |
| D-b-Tocopherol |
| -Tocopherol-d3 |
| Tocopherol, beta- |
| (2R(2R*(4R*,8R*)))-3,4-Dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| (2R)-3,4-DIHYDRO-2,5,8-TRIMETHYL-2-((4R,8R)-4,8,12-TRIMETHYLTRIDECYL)-2H-1-BENZOPYRAN-6-OL |
| (+/-)-beta-Tocopherol |
| DL-BETA TOCOPHEROL |
| (R,R,R)-beta-Tocopherol |
| D-.BETA.-TOCOPHEROL |
| BIDD:PXR0140 |
| SCHEMBL39202 |
| DL-.BETA.-TOCOPHEROL |
| 6-Chromanol, 2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)- |
| RRR-.BETA.-TOCOPHEROL |
| rac- beta -Tocopherol solution |
| .BETA.-TOCOPHEROL [MI] |
| .BETA.-TOCOPHEROL, DL- |
| CHEBI:47771 |
| BETA TOCOPHEROL [WHO-DD] |
| DTXSID10873424 |
| DTXSID30884931 |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl- 2-(4,8,12-trimethyltridecyl)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)- |
| 48223-98-9 |
| VITAMIN E (BETA TOCOTRIENOL) |
| AKOS022171754 |
| AS-74577 |
| HY-133680 |
| J236.825K |
| CS-0128997 |
| J1.282.204I |
| J1.604.281A |
| E76237 |
| Q155667 |
| J-010328 |
| RRR-.ALPHA.-TOCOPHEROL IMPURITY B [EP IMPURITY] |
| FA7BA838-41F9-4B85-947D-F3A920942587 |
| (2R)-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-chromen-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-, ()- |
| (2R)-3,4-Dihydro-2,5,8-trimethyl-2-[(4R,8R)-4,8,12-trimethyltri-decyl]-2H-1-benzopyran-6-ol |
| ?[2R[2R*(4R*,8R*)]]-3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl- 2-(4,8,12-trimethyltridecyl)-, [2R-[2R(4R,8R)]]- |
| 2H-1-BENZOPYRAN-6-OL, 3,4-DIHYDRO-2,5,8-TRIMETHYL-2-(4,8,12-TRIMETHYLTRIDECYL)-, (2R*(4R*,8R*))- 6-CHROMANOL, 2,5,8-TRIMETHYL-2-(4,8,12-TRIMETHYLTRIDECYL)- |
| 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-, [2R-[2R*(4R*,8R*)]]- |
| RAC-(2R*)-3,4-DIHYDRO-2,5,8-TRIMETHYL-2-((4R*,8R*)-4,8,12-TRIMETHYLTRIDECYL)-2H-1-BENZOPYRAN-6-OL |
| REL-(2R*)-2,5,8-TRIMETHYL-2-((4R*,8R*)-4,8,12-TRIMETHYLTRIDECYL)-3,4-DIHYDRO-2H-1-BENZOPYRAN-6-OL |
|
There are more than 10 synonyms. If you wish to see them all click here.
|