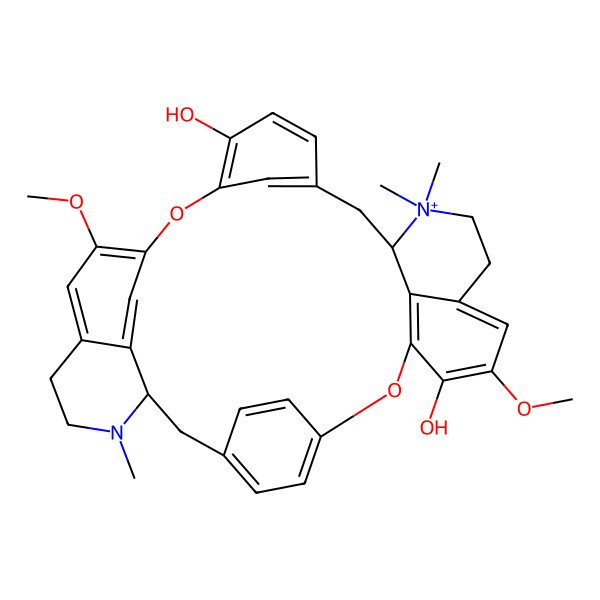
| d-Tubocurarine |
| Tubocurarine chloride |
| Tubocurarin |
| (+)-Tubocurarine |
| Tubocurarinum |
| 57-95-4 |
| Delacurarine |
| Tubarine |
| Isoquinoline alkaloid |
| Jexin |
| D-Tubocurarine chloride |
| Tubocurarine ion |
| Tubocurarine cation |
| CHEBI:9774 |
| Tubocurarine hydrochloride |
| Dextrotubocurarine chloride |
| (+)-Tubocurarine chloride |
| Tubocurarina cloruro |
| UNII-W9YXS298BM |
| Tubocurarini chloridum |
| 7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium |
| W9YXS298BM |
| Cloruro de tubocurarina |
| Chlorure de tubocurarine |
| tubocurarine HCl |
| HSDB 2152 |
| Amerizol |
| Tubaine (TN) |
| Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyl- |
| Tubocurarine chloride (TN) |
| 6989-98-6 |
| (+)-Tubocurarine dichloride |
| (+)-Tubocurarine chloride hydrochloride |
| Tubocurarine chloride (INN) |
| Tubocurarine chloride [INN] |
| C37H41N2O6.ClH.Cl |
| Tubocurarine chloride anhydrous |
| 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraran-2'-ium |
| (+) Tubocurarine |
| 13H-4,6:21,24-Dietheno-8,12-metheno-1H-pyrido(3',2':14,15)(1,11)dioxacycloeicosino(2,3,4-ij)isoquinolinium, 2,3,13a,14,15,16,25,25a-octahydro-9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl-, (13aR,25aS)- |
| 13H-4,6:21,24-Dietheno-8,12-metheno-1H-pyrido(3',2':14,15)(1,11)dioxacycloeicosino(2,3,4-ij)isoquinolinium, 2,3,13a,14,15,16,25,25a-octahydro-9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl-, (13aR-(13aR*,25aS*))- |
| C37H41ClN2O6.ClH.5H2O |
| C37-H41-N2-O6.Cl-H.Cl |
| Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyl-, chloride, hydrochloride |
| NCGC00163242-01 |
| Tubocurarine chloride [USAN:INN:BAN:JAN] |
| C37-H41-N2-O6.Cl-H.Cl.5H2-O |
| Metubine (TN) |
| Tubarine (TN) |
| TC9 |
| Delacurarine (TN) |
| Tubocurarinum (TN) |
| Spectrum_001966 |
| 2,2',2'-trimethyl-6,6'-bis(methyloxy)tubocuraran-2,2'-diium-7',12'-diol dichloride |
| SpecPlus_000475 |
| Jex (TN) |
| 13H-4,6:21,24-Dietheno-8, 12-metheno-1H-pyrido[3',2':14,15][1,11]dioxacycloeicosino [2,3,4-ij]isoquinolinium, 2,3,13a,14,15,16,25,25a-octahydro- 9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl -, chlorid |
| 57-94-3 |
| Spectrum2_001335 |
| Spectrum3_001095 |
| Spectrum4_001922 |
| Spectrum5_000685 |
| Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2,2',2'-tetramethyl-, dichloride, pentahydrate |
| D05HSC |
| Epitope ID:174836 |
| TUBOCURARINE [HSDB] |
| dimethoxy(trimethyl)[?]diol |
| TUBOCURARINE [VANDF] |
| BSPBio_002770 |
| KBioGR_002264 |
| KBioSS_002526 |
| MLS003882581 |
| DivK1c_006571 |
| SCHEMBL121375 |
| TUBOCURARINE [WHO-DD] |
| SPBio_001489 |
| CHEMBL339427 |
| GTPL2294 |
| DTXSID0048393 |
| KBio1_001515 |
| KBio2_002518 |
| KBio2_005086 |
| KBio2_007654 |
| KBio3_001990 |
| C37H41N2O6 |
| HMS2089C06 |
| BDBM50366799 |
| PDSP1_001485 |
| PDSP2_001469 |
| C37-H41-N2-O6 |
| DB01199 |
| SDCCGMLS-0066631.P001 |
| NCGC00163242-02 |
| NCGC00178480-01 |
| SMR002533646 |
| SBI-0052455.P002 |
| LS-187260 |
| C07547 |
| AB00053831-03 |
| AB00053831_04 |
| Q421268 |
| SR-05000001878-4 |
| Tubocurarine, chloride, hydrochloride, (+)-(8CI) |
| BRD-K99621550-003-03-4 |
| (1S,16R)-10,25-dimethoxy-15,15,30-trimethyl-7,23-dioxa-30-aza-15-azoniaheptacyclo[22.6.2.23,6.18,12.118,22.027,31.016,34]hexatriaconta-3(36),4,6(35),8(34),9,11,18(33),19,21,24,26,31-dodecaene-9,21-diol |
| (1S,16R)-9,21-dihydroxy-10,25-dimethoxy-15,15,30-trimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2^{3,6}.1^{8,12}.1^{18,22}.0^{27,31}.0^{16,34}]hexatriaconta-3,5,8,10,12(34),18(33),19,21,24(32),25,27(31),35-dodecaen-15-ium |
| 13H-4,6:21,24-Dietheno-8,12-metheno-1H-pyrido[3',2':14,15][1,11]dioxacycloeicosino[2,3,4-ij]isoquinolinium,2,3,13a,14,15,16,25,25a-octahydro-9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl-,(13ar,25a |
|
There are more than 10 synonyms. If you wish to see them all click here.
|