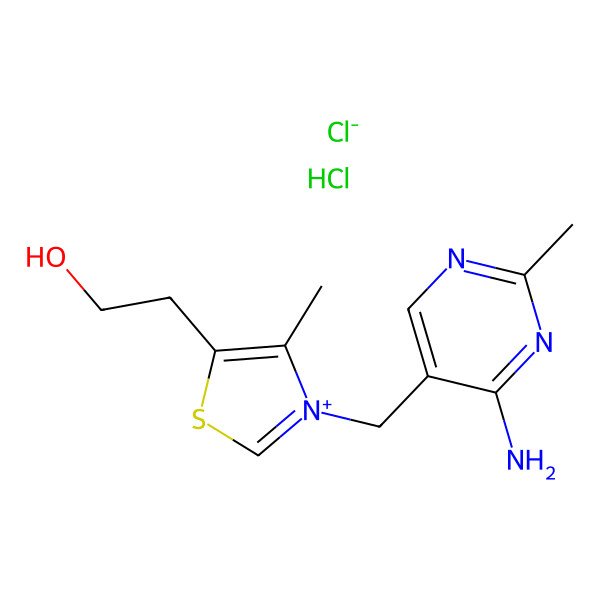
| 67-03-8 |
| Thiamine HCL |
| Aneurine hydrochloride |
| Vitamin B1 hydrochloride |
| Clotiamina |
| Eskaphen |
| Betalin S |
| Thiamine chloride hydrochloride |
| Thiamine dichloride |
| vitamin B1 |
| Begiolan |
| Bethiazine |
| Bevitine |
| Bithiamin |
| Metabolin |
| Tiamidon |
| Tiaminal |
| Beatine |
| Bedome |
| Benerva |
| Beuion |
| Bevitex |
| Bivatin |
| Bivita |
| Eskapen |
| Slowten |
| Thiamol |
| Thiavit |
| Berin |
| Biuno |
| Hybee |
| Apate drops |
| Lixa-beta |
| Thiamin chloride |
| Thiaminum hydrochloricum |
| thiamine(2+) dichloride |
| Thiadoxine |
| Vitaneuron |
| Bequin |
| Thiamin hydrochloride |
| Thiaminal |
| Trophite |
| Vinothiam |
| Betabion hydrochloride |
| Vitamin B hydrochloride |
| Vetalin S |
| FEMA No. 3322 |
| Thiamin dichloride |
| Thiaminium chloride |
| Thiaminium chloride hydrochloride |
| Betaxin |
| Bewon |
| Thiamine ion |
| Usaf cb-20 |
| Vitamin b(sub 1) hydrochloride |
| Thiamine, monohydrochloride |
| MFCD00012780 |
| NSC-36226 |
| thiamine |
| thymine hydrochloride |
| CCRIS 1906 |
| Thiamine monohydrochloride |
| DTXSID0040622 |
| UNII-M572600E5P |
| CHEBI:49105 |
| Thiamine, hydrochloride |
| Thiamine (hydrochloride) |
| EINECS 200-641-8 |
| NSC 36226 |
| 3-((4-amino-2-methylpyrimidin-5-yl)methyl)-5-(2-hydroxyethyl)-4-methylthiazol-3-ium chloride hydrochloride |
| M572600E5P |
| Thiamine HCl (Vitamin B1) |
| Vitamin B1 (Thiamine HCl) |
| AI3-18993 |
| 67-03-8 (HCl salt) |
| Thiamine chloride, hydrochloride |
| DTXCID8020622 |
| Thiamine hydrochloride [USP:JAN] |
| Thiamine Hydrochloride (Vitamin B1) |
| CAS-67-03-8 |
| Vitamin B1 hydrochloride (VAN) |
| NCGC00017013-01 |
| Thiamine, chloride, hydrochloride |
| Vitamin b(sup1) |
| NSC36226 |
| 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol chloride hydrochloride |
| 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol;chloride;hydrochloride |
| 3-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methylthiazolium chloride monohydrochloride |
| 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium chloride hydrochloride |
| Thiamine hydrochloride 10 microg/mL in Acetonitrile |
| Thiamine chloride hydrochloride;Vitamin B1 hydrochloride |
| UNII-4ABT0J945J |
| THIAMINE HYDROCHLORIDE (MART.) |
| THIAMINE HYDROCHLORIDE [MART.] |
| THIAMINE HYDROCHLORIDE (USP-RS) |
| THIAMINE HYDROCHLORIDE [USP-RS] |
| SMR000875246 |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl- chloride, monohydrochloride |
| THIAMINE HYDROCHLORIDE (EP IMPURITY) |
| THIAMINE HYDROCHLORIDE [EP IMPURITY] |
| THIAMINE HYDROCHLORIDE (EP MONOGRAPH) |
| THIAMINE HYDROCHLORIDE [EP MONOGRAPH] |
| THIAMINE HYDROCHLORIDE (USP MONOGRAPH) |
| THIAMINE HYDROCHLORIDE [USP MONOGRAPH] |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-, chloride, monohydrochloride |
| vitamin Bl |
| 3-((4-amino-2-methylpyrimidin-5-yl)methyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium chloride hydrochloride |
| 3-((4-azaniumyl-2-methylpyrimidin-5-yl)methyl)-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium dichloride |
| 3-[(4-azaniumyl-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium dichloride |
| Thiazolium, 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl- chloride, monohydrochloride |
| Aneurinehydrochloride |
| Prestwick_441 |
| Thiamini hydrochloridum |
| Thiamine HCL (TN) |
| Vitamin B1hydrochloride |
| Thiazolium, hydrochloride |
| 3-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m- ethylthiazolium chloride, monohydrochloride |
| 3-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m-ethylthiazolium chloride, monohydrochloride |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m- ethyl, chloride, monohydrochloride |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-m-ethyl, chloride, monohydrochloride |
| D02LDV |
| VITAMIN B1 [FHFI] |
| THIAMINE HCL [INCI] |
| Thiazolium, monohydrochloride |
| C12H17N4OS.ClH.Cl |
| SCHEMBL41101 |
| MLS001332447 |
| MLS001332448 |
| Thiamine hydrochloride (USP) |
| Aneurine Hydrochloride Hydrate |
| Thiamine for system suitability |
| CHEMBL1200941 |
| Vitamin B1 Hydrochloride Hydrate |
| DPJRMOMPQZCRJU-UHFFFAOYSA-M |
| HMS1569P04 |
| THIAMINE HYDROCHLORIDE [MI] |
| BCP27971 |
| HY-N0680 |
| THIAMINE HYDROCHLORIDE [FCC] |
| Tox21_110736 |
| s3211 |
| Thiamine hydrochloride, p.a., 98% |
| C12-H17-N4-O-S.Cl-H.Cl |
| THIAMINE HYDROCHLORIDE [VANDF] |
| AKOS015905506 |
| THYMINE HYDROCHLORIDE [WHO-DD] |
| CCG-220677 |
| CS-8164 |
| SB57886 |
| THIAMINE HYDROCHLORIDE [WHO-DD] |
| THIAMINE HYDROCHLORIDE [WHO-IP] |
| THIAMINUM HYDROCHLORICUM [HPUS] |
| Thiamine chloride hydrochloride (JP17) |
| NCGC00017013-02 |
| 2-{3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-5-yl}ethan- 1-ol, chloride, chloride |
| AS-14151 |
| SY010871 |
| THIAMINE HYDROCHLORIDE [ORANGE BOOK] |
| Thiamine hydrochloride, >=98%, FCC, FG |
| FT-0631293 |
| FT-0777960 |
| T0181 |
| THIAMINE CHLORIDE HYDROCHLORIDE [JAN] |
| THIAMINI HYDROCHLORIDUM [WHO-IP LATIN] |
| D02094 |
| EN300-258109 |
| Thiamine Hydrochloride (B1), analytical standard |
| THIAMINE CHLORIDE, HYDROCHLORIDE [WHO-IP] |
| Q-201928 |
| Thiamine hydrochloride, tested according to Ph.Eur. |
| Q27121486 |
| Thiamine hydrochloride, reagent grade, >=99% (HPLC) |
| Thiamine hydrochloride, SAJ special grade, >=98.5% |
| F0001-2415 |
| F2173-0852 |
| Thiamine hydrochloride, meets USP testing specifications |
| Z1954805523 |
| WLN: T6N CNJ B1 DZ E1- AT5K CSJ D2Q E1 &Q &G &GH |
| Thiamine hydrochloride, 99% (dry wt.), may cont. up to 5% water |
| Thiamine Hydrochloride (Vitamin B1) 1.0 mg/ml in Methanol (as free base) |
| Thiamine hydrochloride, European Pharmacopoeia (EP) Reference Standard |
| Thiamine hydrochloride, United States Pharmacopeia (USP) Reference Standard |
| Thiamine for system suitability, European Pharmacopoeia (EP) Reference Standard |
| Thiamine hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material |
| THIAZOLIUM,3-[(4-AMINO-2-METHYL-5-PYRIMIDINYL)METHYL]-5-(2-HYDROXYETHYL)-4-METHYL- |
| 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium hydrochloride chloride |
| Thiamine hydrochloride, BioReagent, suitable for cell culture, suitable for insect cell culture, suitable for plant cell culture |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-, chloride, hydrochloride (1:1:1) |
| Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-chloride, monohydrochloride |
| Thiazolium, 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl- chloride, monohydrochloride |
| Tiazolio, 3-[(4-amino-2-metil-5-pirimidinil) metil]-5-(2-hidroxietil)-4-metil-cloruro (1:1), clorhidrato (1:1) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|