| Teslac |
| Teolit |
| 968-93-4 |
| Fludestrin |
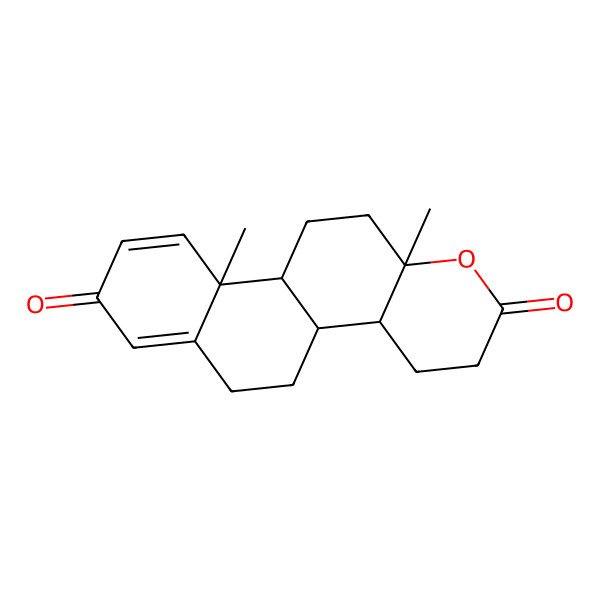
| Testolacton |
| 1-Dehydrotestololactone |
| 1,2-Didehydrotestololactone |
| delta(1)-Testololactone |
| Testolattone [DCIT] |
| Testolactonum [INN-Latin] |
| Testolactona [INN-Spanish] |
| Testolactona |
| Testolactonum |
| Testolattone |
| D-Homo-17A-oxaandrosta-1,4-diene-3,17-dione |
| SQ 9538 |
| SQ-9538 |
| Testololactone, 1-dehydro- |
| delta(1)-Dehydrotestolactone |
| delta(1)-Testolactone |
| 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone |
| Testolactone ciii |
| Testolactone [USAN:INN] |
| NSC-23759 |
| Teslak |
| delta1-Testololactone |
| Testololactone, 1,2-didehydro- |
| Delta-1-testololactone |
| HSDB 3255 |
| EINECS 213-534-6 |
| TESLAC (TN) |
| Testolactone (USP/INN) |
| NSC 23759 |
| NSC-12173 |
| 1,2-Dehydrotestololactone |
| (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-decahydro-2H-naphtho[2,1-f]chromene-2,8(4bH)-dione |
| 17alpha-Oxo-D-homo-1,4-androstadiene-3,17-dione |
| UNII-6J9BLA949Q |
| 3-oxo-13,17-secoandrosta-1,4-dieno-17,13alpha-lactone |
| 6J9BLA949Q |
| CHEBI:9460 |
| Testolactone [USAN:USP:INN] |
| DTXSID2023644 |
| (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-4,4a,4b,5,6,10b,11,12-octahydro-3H-naphtho[2,1-f]chromene-2,8-dione |
| 1,2,3,4,4a,4b,7,9,10,10a-Decahydro-2-hydroxy-2,4b-dimethyl-7-oxo-1-phenanthrenepropionic acid delta-lactone |
| 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone |
| NSC 12173 |
| 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, .delta.-lactone |
| (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-4,4a,4b,5,6,10a,10b,11,12,12a-decahydro-2H-naphtho[2,1-f]chromene-2,8(3H)-dione |
| 1 Dehydrotestolactone |
| 1-Dehydrotestolactone |
| .DELTA.1-Testololactone |
| .DELTA.1-Dehydrotestolactone |
| .DELTA.1-Dehydrotestololactone |
| NSC23759 |
| NCGC00159329-02 |
| delta-1-testolactone |
| therapeutic testolactone |
| 17a-Oxa-D-homoandrosta-1,4-diene-3,17-dione |
| delta1-Dehydrotestolactone |
| TESTOLACTONE [MI] |
| TESTOLACTONE [INN] |
| D0C7JF |
| delta1-Dehydrotestololactone |
| TESTOLACTONE [HSDB] |
| TESTOLACTONE [USAN] |
| SCHEMBL4053 |
| Testololactone,2-didehydro- |
| CHEMBL1571 |
| TESTOLACTONE [VANDF] |
| TESTOLACTONE [USP-RS] |
| TESTOLACTONE [WHO-DD] |
| DTXCID303644 |
| GTPL7303 |
| 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid .delta.-lactone |
| HMS3750O07 |
| TESTOLACTONE [ORANGE BOOK] |
| TESTOLACTONE [USP IMPURITY] |
| TESTOLACTONE CIII [USP-RS] |
| BCP10926 |
| Tox21_111576 |
| BDBM50367848 |
| LMST02020084 |
| 17a-Oxa-D-homoandrosta-1,17-dione |
| D-Homo-17a-oxaandrosta-1,17-dione |
| AKOS015840139 |
| CS-5268 |
| DB00894 |
| Bristol Myers SquibbBrand of Testolactone |
| CAS-968-93-4 |
| HY-13763 |
| NCI60_001908 |
| Bristol-Myers Squibb Brand of Testolactone |
| LS-102968 |
| C02197 |
| D00153 |
| D-homo-17a-oxaandros-ta-1,4-diene-3,17-dione |
| Q3985253 |
| W-100129 |
| 13,4-dien-17-oic acid, 13-hydroxy-3-oxo-, lactone |
| 13-Hydroxy-3-oxo-13,4-dien-17-oic acid .delta.-lactone |
| 13,4-dien-17-oic acid, 13-hydroxy-3-oxo-, .delta.-lactone |
| 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid lactone |
| 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, lactone |
| 13,4-dien-17-oic acid, 13.alpha.-hydroxy-3-oxo-, .delta.-lactone |
| 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone (8CI) |
| 13,17-Secoandrosta-1,4-dien-17-oic acid, 13.alpha.-hydroxy-3-oxo-, .delta.-lactone |
| 2H-Phenanthro[2,1-b]pyran-2,8(4bH)-dione, 3,4,4a,5,6,10a,10b,11,12,12a-decahydro-10a,12a-dimethyl-, lactone |
| 2H-Phenanthro[2,8(4bH)-dione, 3,4,4a,5,6,10a,10b,11,12,12a-decahydro-10a,12a-dimethyl-, lactone |
|
There are more than 10 synonyms. If you wish to see them all click here.
|