| Counter |
| 13071-79-9 |
| Contraven |
| Counter 15G soil insecticide |
| AC 92100 |
| ENT 27920 |
| Counter 15G |
| Counter 15G soil insecticide-nematicide |
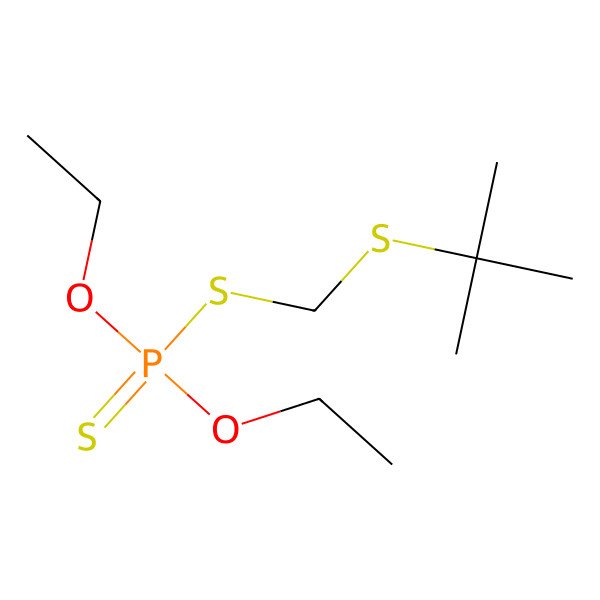
| S-tert-Butylthiomethyl O,O-diethyl phosphorodithioate |
| Phosphorodithioic acid S-((tert-butylthio)methyl) O,O-diethyl ester |
| Phosphorodithioic acid, O,O-diethyl S-(((1,1-dimethylethyl)thio)methyl) ester |
| Phosphorodithioic acid, S-[[(1,1-dimethylethyl)thio]methyl] O,O-diethyl ester |
| M83BN0F8R9 |
| DTXSID2022254 |
| CHEBI:38960 |
| S-(((1,1-Dimethylethyl)thio)methyl) O,O-diethyl phosphorodithioate |
| S-(((1,1-Dimethylethyl)thio)methyl)-O,O-diethyl phosphorodithioate |
| S-t-Butylthio-methyl-O,O-diethyl phosphorodithioate |
| Aragran |
| Phosphorodithioic acid, S-[(tert-butylthio)methyl] O,O-diethyl ester |
| DTXCID702254 |
| O,O-Diethyl S-(Tert-butylthio)methyl phosphorodithioate |
| S-[(tert-butylthio)methyl] O,O-diethyl dithiophosphate |
| S-[(tert-Butylsulfanyl)methyl] o,o-diethyl dithiophosphate |
| Caswell No. 131A |
| S-[(tert-butylsulfanyl)methyl] O,O-diethyl phosphorodithioate |
| O,O-diethyl S-(((1,1-dimethylethyl)thio)methyl)phoshorodithioate |
| Phosphorodithioic acid, S-(((1,1-dimethylethyl)thio)methyl) O,O-diethyl ester |
| Terbufos [ANSI:BSI:ISO] |
| Terbufos [ISO] |
| CAS-13071-79-9 |
| CCRIS 4772 |
| HSDB 6444 |
| St-100 |
| AI3-27920 |
| EINECS 235-963-8 |
| EPA Pesticide Chemical Code 105001 |
| BRN 1710115 |
| UNII-M83BN0F8R9 |
| Cyanater |
| S-tert-Butylthiomethyl O,O-diethylphosphorodithioate |
| S-((tert-Butylthio)methyl)O,O-diethylphosphorodithioate |
| TERBUFOS [HSDB] |
| TERBUFOS [MI] |
| Phosphorodithioic acid S-(((1,1-dimethylethyl)thio)methyl) O,O-diethyl ester |
| SCHEMBL23773 |
| 4-01-00-03092 (Beilstein Handbook Reference) |
| CHEMBL1406292 |
| Tox21_201634 |
| Tox21_302994 |
| Terbufos 10 microg/mL in Cyclohexane |
| Terbufos 1000 microg/mL in Acetone |
| AKOS016014230 |
| Terbufos 10 microg/mL in Acetonitrile |
| Terbufos 100 microg/mL in Acetonitrile |
| NCGC00091771-01 |
| NCGC00091771-02 |
| NCGC00091771-03 |
| NCGC00256426-01 |
| NCGC00259183-01 |
| AC-92100 |
| O,O-Diethyl-S-1,1-dimethylethylthiomethyl |
| Terbufos, PESTANAL(R), analytical standard |
| C18693 |
| J-005860 |
| Q2404344 |
| S-[(tert-Butylthio)methyl] O,O-diethyl phosphorodithioate |
| S-[(tert-Butylsulfanyl)methyl] O,O-diethyl dithiophosphate # |
| O,O-Diethyl S-[[(1,1-dimethylethyl)thio]methyl] phosphorodithioate |
| S-[[(1,1-Dimethylethyl)thio]methyl] O,O-diethyl phoshporodithioate |
| tert-butylsulfanylmethylsulfanyl-diethoxy-sulfanylidene-lambda5-phosphane |
| Methanethiol, (tert-butylthio)-, S-ester with O,O-diethyl phosphorodithioate |
|
There are more than 10 synonyms. If you wish to see them all click here.
|