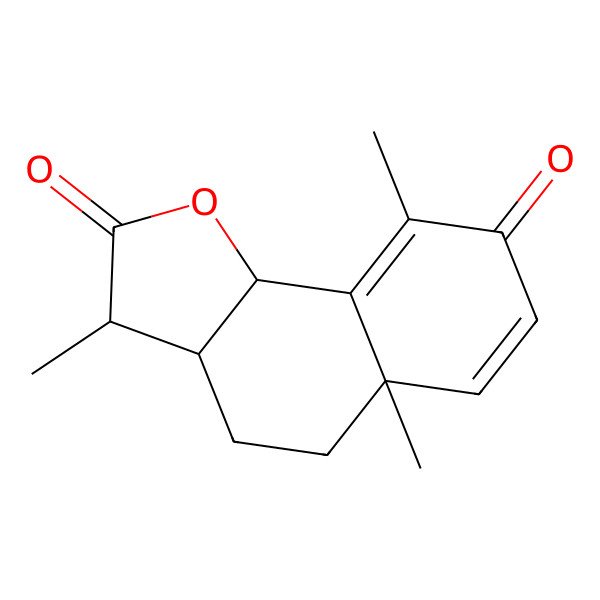
| alpha-Santonin |
| 481-06-1 |
| (-)-alpha-Santonin |
| Semenen |
| Santoninum |
| Santoninic anhydride |
| L-alpha-Santonin |
| (-)-Santonine |
| (-)-Santonin |
| a-Santonin |
| Santonin [JAN] |
| Santonin [JAN:NF] |
| NSC4900 |
| NSC-4900 |
| UNII-1VL8J38ERO |
| 1VL8J38ERO |
| DTXSID7045312 |
| CHEBI:16363 |
| .alpha.-Santonin |
| EINECS 207-560-7 |
| NSC 41311 |
| (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,4,5,9b-tetrahydro-3H-benzo[g][1]benzofuran-2,8-dione |
| (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,5,5a,9b-tetrahydronaphtho[1,2-b]furan-2,8(3H,4H)-dione |
| AI3-19471 |
| DTXCID5025312 |
| NSC 4900 |
| l-.alpha.-Santonin |
| 11-Epiisoeusantona-1,4-dienic acid, 6alpha-hydroxy-3-oxo-, gamma-lactone |
| (3S)-2,3,3a,4,5,5a,8,9bbeta-Octahydro-3,5abeta,9-trimethylnaphtho(1,2-b)furan-2,8-dion |
| (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,4,5,9b-tetrahydro-3H-benzo[g]benzofuran-2,8-dione |
| Eudesma-1,4-dien-12-oic acid, 6-alpha-hydroxy-3-oxo-, gamma-lactone, (11S)-(-)- |
| Naphtho(1,2-b)puran-2,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl- |
| naphtho[1,2-b]furan-2,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl-, (3S,3aS,5aS,9bS)- |
| (-)-.alpha.-Santonin |
| SR-01000635568 |
| -santonin |
| NCGC00016461-01 |
| Santonin (TN) |
| (3S,3aS,5aS,9bS)-2,3,3a,4,5,5a,8,9b-Octahydro-3,5a,9-trimethylnaphtho(1,2-b)furan-2,8-dione |
| (3S,3aS,5aS,9bS)-2,3,3a,4,5,5a,8,9b-octahydro-3,5a,9-trimethylnaphtho[1,2-b]furan-2,8-dione |
| (3S,3AS,5AS,9BS)-3A,5,5A,9B-TETRAHYDRO-3,5A,9-TRIMETHYLNAPHTHO(1,2-B)FURAN-2,8(3H,4H)-DIONE |
| (3S,3aS,5aS,9bS)-3a,5,5a,9b-Tetrahydro-3,5a,9-trimethylnaphtho[1,2-b]furan-2,8(3H,4H)-dione |
| CAS-481-06-1 |
| Naphtho(1,2-b)furan-2,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl-, (3S,3aS,5aS,9bS)- |
| Naphtho(1,2-b)furan-2,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl-, (3S-(3alpha,3aalpha,5abeta,9bbeta))- |
| ()-alpha-Santonin |
| MFCD00135865 |
| SANTONINE |
| Santonin (JP17) |
| Spectrum_000790 |
| SpecPlus_000318 |
| (-)- alpha -Santonin |
| Prestwick0_001070 |
| Prestwick1_001070 |
| Prestwick2_001070 |
| Prestwick3_001070 |
| Spectrum2_000699 |
| Spectrum3_001245 |
| Spectrum4_001476 |
| Spectrum5_000151 |
| SANTONINUM [HPUS] |
| SANTONIN [MART.] |
| SANTONIN [WHO-DD] |
| UPCMLD-DP084 |
| (3S,5aS,9bS)-3a,5,5a,9b-Tetrahydro-3,5a,9-trimethylnaphtho[1,2-b]furan-2,8(3H,4H)dione |
| BSPBio_001060 |
| BSPBio_002750 |
| KBioGR_002051 |
| KBioSS_001270 |
| SPECTRUM300542 |
| MLS002154141 |
| DivK1c_006414 |
| SPBio_000857 |
| SPBio_002970 |
| alpha-Santonin; (-)-Santonin |
| BPBio1_001166 |
| CHEMBL259254 |
| MEGxp0_001636 |
| SCHEMBL1133565 |
| .ALPHA.-SANTONIN [MI] |
| UPCMLD-DP084:001 |
| GTPL12448 |
| KBio1_001358 |
| KBio2_001270 |
| KBio2_003838 |
| KBio2_006406 |
| KBio3_002250 |
| (-)-alpha-Santonin, >=99% |
| Eudesma-1,4-dien-12-oic acid, 6-alpha-hydroxy-3-oxo-, gamma-lactone, (11S)- |
| HMS1571E22 |
| HMS2098E22 |
| HMS2268H12 |
| HY-B1761 |
| Tox21_110445 |
| CCG-40021 |
| s3999 |
| AKOS015895177 |
| Tox21_110445_1 |
| LMPR0103190001 |
| SDCCGMLS-0066491.P001 |
| (-)-alpha-Santonin, analytical standard |
| NCGC00161640-01 |
| NCGC00161640-02 |
| NCGC00161640-03 |
| NCGC00263447-01 |
| SMR000112520 |
| AB00376930 |
| CS-0013789 |
| S0521 |
| EN300-34530 |
| C02206 |
| D00154 |
| WLN: T B566 COV LV IHTT&J E1 I1 M1 |
| A827469 |
| Q413166 |
| SR-01000635568-1 |
| SR-01000635568-4 |
| SR-01000635568-5 |
| BRD-K58787433-001-05-4 |
| BRD-K58787433-001-08-8 |
| BRD-K58787433-001-12-0 |
| Santonin, European Pharmacopoeia (EP) Reference Standard |
| Z359371114 |
| 11-Epiisoeusantona-1, 6.alpha.-hydroxy-3-oxo-, .gamma.-lactone |
| 3-(6-NITRO-2-OXO-1,3-BENZOXAZOL-3(2H)-YL)PROPANOICACID |
| Eudesma-1, 6.alpha.-hydroxy-3-oxo-, .gamma.-lactone, (11S)- |
| (11S)-6alpha-hydroxy-3-oxoeudesma-1,4-dien-12-oic acid gamma-lactone |
| 6alpha-hydroxy-3-oxo-11-epiisoeusantona-1,4-dienic acid gamma-lactone |
| Eudesma-1, 6.alpha.-hydroxy-3-oxo-, .gamma.-lactone, (11S)-(-)- |
| Naphtho[1,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl- |
| 3a,5,5a,9b-Tetrahydro-3,5a,9-trimethyl-naphtho[1,2-b]puran-2,8(3H,4H)-dione |
| (3S,3AS,5AS)-3,5A,9-TRIMETHYL-3A,4,5,5A-TETRAHYDRONAPHTHO(1,2-B)FURAN-2,8(3H,9BH)-DIONE |
| (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-2H,3H,3aH,4H,5H,5aH,8H,9bH-naphtho[1,2-b]furan-2,8-dione |
| (3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,4,5,5a-tetrahydronaphtho[1,2-b]furan-2,8(3H,9bH)-dione |
| [3S-(3alpha,3aalpha,5abeta,9bbeta)]-3a,5,5a,9b-Tetrahydro-3,5a,9-trimethylnaphtho[1,2-b]furan-2,8(3H,4H)-dione |
| 1,2,3,4,4a,7-hexahydro-1-hydroxy-alpha,4a,8-trimethyl-7-oxo-2-naphthaleneacetic acid gamma-lactone |
| 1,3,4,4a,7-Hexahydro-1-hydroxy-.alpha., 4a,8-trimethyl-7-oxo-2-naphthaleneacetic acid .gamma.-lactone |
| InChI=1/C15H18O3/c1-8-10-4-6-15(3)7-5-11(16)9(2)12(15)13(10)18-14(8)17/h5,7-8,10,13H,4,6H2,1-3H3/t8-,10-,13-,15-/m0/s |
| Naphtho[1,8(3H,4H)-dione, 3a,5,5a,9b-tetrahydro-3,5a,9-trimethyl-, [3S-(3.alpha.,3a.alpha.,5a.beta.,9b.beta.)]- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|