| 52645-53-1 |
| Ambush |
| Transpermethrin |
| Pounce |
| Elimite |
| 1RS,cis-Permethrin |
| Permethrine |
| Permethrinum |
| Acticin |
| Corsair |
| Dragnet |
| Ectiban |
| Imperator |
| Kestrel |
| Lyclear |
| Outflank |
| Perigen |
| Permasect |
| Permetrina |
| Perthrine |
| Quamlin |
| Stomoxi |
| Stomoxin |
| Coopex |
| Cosair |
| Dragon |
| Eksmin |
| Picket |
| Exmin |
| Expar |
| Kafil |
| Kavil |
| Kudos |
| 1RS-trans-Permethrin |
| Transpermethrin [ISO] |
| Ridect pour-on |
| (+-)-cis-Permethrin |
| Insorbcid MP |
| Perigen W |
| Permethrin,racemic |
| trans-(+-)-Permethrin |
| Mitin BC |
| Permanone 80 |
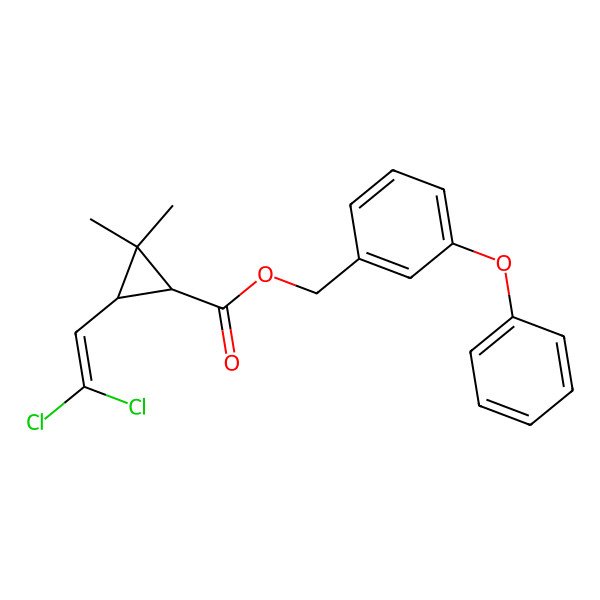
| (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate |
| Chinetrin |
| Ecsumin |
| Efmethrin |
| Indothrin |
| NRDC 146 |
| NRDC 148 |
| Exsmin |
| Ipitox |
| Caswell No. 652BB |
| 52341-32-9 |
| (+-)-trans-Permethrin |
| Permethrine,c&t |
| (+-)-cis-Fmc 33297 |
| Diffusil H |
| Stomoxin P |
| Outflank-stockade |
| Dragnet FT |
| Picket G |
| Activyl tick plus |
| Permasect-25EC |
| FMC 35171 |
| Permit |
| Pramex |
| Kestrel (pesticide) |
| UNII-509F88P9SZ |
| Antiborer 3768 |
| Bematin 987 |
| CCRIS 2001 |
| ICI-PP 557 |
| NRDC 143 |
| SBP 15131TEC |
| Permethrinum [Latin] |
| DTXSID8022292 |
| CHEBI:34911 |
| Permetrin (Hungarian) |
| HSDB 6790 |
| Permitrene (Hungarian) |
| Permetrina [Portuguese] |
| trans-Permethrin D6 (dimethyl D6) |
| EINECS 258-067-9 |
| FMC 33297 |
| FMC 41655 |
| NIA 33297 |
| PP 557 |
| Hemoglobin atlanta-coventry |
| Permethrine [ISO-French] |
| EPA Pesticide Chemical Code 109701 |
| NSC-760105 |
| JF 7065 |
| BRN 2063148 |
| BRN 4153590 |
| (+)-trans-Permethrin |
| Ambushfog |
| Kaleait |
| Stomozan |
| WL 43479 |
| SBP-1513TEC |
| Permethrin [USAN:INN:BAN] |
| AI3-29296 |
| Anomethrin N |
| DTXCID102292 |
| MP79 |
| BW-21-Z |
| S 3151 |
| 509F88P9SZ |
| OMS 1821 |
| 1RS cis-Permethrin |
| 3-phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| C21H20Cl2O3 |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropane carboxylic acid, (3-phenoxyphenyl) methyl ester |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester |
| NRDC-143 |
| Permethrinum (Latin) |
| NSC 760105 |
| NCGC00159390-02 |
| AI3-29158 |
| (+-)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-Phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate |
| m-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester |
| 3-Phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| 82523-59-9 |
| Cooper |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, cis-(+-)- |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, trans-(+-)- |
| Cyclopropanecarboxylicacid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester,(1R,3S)-rel- |
| Permethrin (ANSI:BSI:ISO) |
| Permethrin [ANSI:BSI:ISO] |
| NRDC 147 |
| S-3151 |
| PerFoam |
| SBP-1513 |
| Acticin Cream |
| Elimite Cream |
| Nix Cream Rinse |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate |
| [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-Phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-Phenoxybenzyl(+-)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylate |
| m-Phenoxybenzyl (+-)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| m-Phenoxybenzyl (+1)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| (3-Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-(Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| 52341-33-0 |
| 93389-07-2 |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-,(3-phenoxyphenyl)methyl ester, (1R,3R)-rel- |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-,(3-phenoxyphenyl)methyl ester, (1R,3S)-rel- |
| Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, 3-phenoxybenzyl ester, (+-)-, (cis,trans)- |
| SMR000778043 |
| LE 79-519 |
| CAS-52645-53-1 |
| C21-H20-Cl2-O3 |
| Permethrn |
| 3-Phenoxybenzyl-cis,trans-(1RS)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Permethrin, (cis-(+-))-Isomer |
| Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R-trans)- |
| Permethrin, (trans-(+-))-Isomer |
| RU 22090 |
| Hb Atlanta-coventry |
| Elimite (TN) |
| AI3-29190 |
| Hb At-Co |
| Permethrin (isomers) |
| ( -)-cis-Permethrin |
| OUTFLANK STOCKADE |
| Permethrin (USAN/INN) |
| PERMETHRIN (IARC) |
| PS758_SUPELCO |
| cispermethrin (cis isomer) |
| D0K8MP |
| PERMETHRIN (MART.) |
| 3-Phenoxybenzyle (1RS)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylate |
| biopermethrin (trans isomer) |
| CHEMBL1525 |
| SCHEMBL26543 |
| MLS001332525 |
| MLS001332526 |
| Permethrin [USAN:BAN:INN] |
| Permethrin cis/trans ~ 1:1 |
| Permethrin, analytical standard |
| NDRC 143 |
| 3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| SCHEMBL15218274 |
| BW 21-Z |
| P03AC04 |
| RLLPVAHGXHCWKJ-UHFFFAOYSA-N |
| HMS2232L22 |
| HMS3264N07 |
| HMS3369D10 |
| Pharmakon1600-01504932 |
| (3-Phenoxyphenyl)methyl (+-)cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| (3-phenoxyphenyl)methyl (1R,3S)-3-(2,2-dichloroethenyl)-2,2-bis(trideuteriomethyl)cyclopropane-1-carboxylate |
| HY-B0887 |
| SBP 1513 |
| Tox21_111627 |
| Tox21_201586 |
| Tox21_300691 |
| MFCD00041809 |
| NSC760105 |
| s6461 |
| STL135986 |
| COMPONENT OF ACTIVYL TICK PLUS |
| VECTRA 3D COMPONENT PERMETHRIN |
| AKOS005746953 |
| CCG-213703 |
| CS-O-10185 |
| DB04930 |
| KS-5079 |
| Permethrin 1000 microg/mL in Acetone |
| PERMETHRIN (EMA EPAR VETERINARY) |
| Permethrin (including cis- and trans-) |
| Permethrin 10 microg/mL in Cyclohexane |
| USEPA/OPP Pesticide Code: 109701 |
| NCGC00159390-00 |
| NCGC00159390-04 |
| NCGC00159390-05 |
| NCGC00159390-06 |
| NCGC00159390-07 |
| NCGC00159390-08 |
| NCGC00159390-09 |
| NCGC00159390-10 |
| NCGC00159390-11 |
| NCGC00159390-12 |
| NCGC00159390-13 |
| NCGC00159390-14 |
| NCGC00254599-01 |
| NCGC00259135-01 |
| Permethrin 100 microg/mL in Cyclohexane |
| PERMETHRIN COMPONENT OF VECTRA 3D |
| LS-58636 |
| LS-58640 |
| Permethrin (isomers), analytical standard |
| Total Permethrin 100 microg/mL in Acetone |
| ACTIVYL TICK PLUS COMPONENT PERMETHRIN |
| FT-0630656 |
| Permethrin, PESTANAL(R), analytical standard |
| D05443 |
| AB00918441_05 |
| Permethrin is known as a pyrethroid insecticide. |
| EN300-19628849 |
| MIXTURE OF CIS AND TRANS PERMETHRIN ISOMERS |
| Q411635 |
| J-523915 |
| Permethrin (25:75), EuropePharmacopoeia (EP) Reference Standard |
| (1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propanecarboxylate |
| 3-phenoxybenzyl 2-(2,2-dichlorovinyl)3,3-dimethylcyclopropanecarboxylate |
| (+/-)-3-PHENOXYBENZYL 3-(2,2-DICHLOROVINYL)-2,2-DIMETHYLCYCLOPROPANECARBOXYLATE |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-carboxylate |
| (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| (3-phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropanecarboxylate |
| (3-phenoxyphenyl)methyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-carboxylate |
| 3-phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| M-PHENOXYBENZYL (+/-)-3-(2,2-DICHLOROVINYL)-2,2-DIMETHYLCYCLOPROPANECARBOXYLATE |
| m-phenoxybenzyl 2,2-dimethyl-3-(2',2'-dichlorovinyl)-cyclopropanecarboxylate |
| Permethrin for system suitability, EuropePharmacopoeia (EP) Reference Standard |
| (3-Phenoxyphenyl)methyl (+/-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| 3-(2,2-DICHLOROETHENYL)-2,2-DIMETHYLCYCLOPROPANE CARBOXYLIC ACID, (3-PHENOXY-PHENYL)METHYL ESTER |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid, (3-phenoxyphenyl)methyl ester |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, cis-(.+.)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|