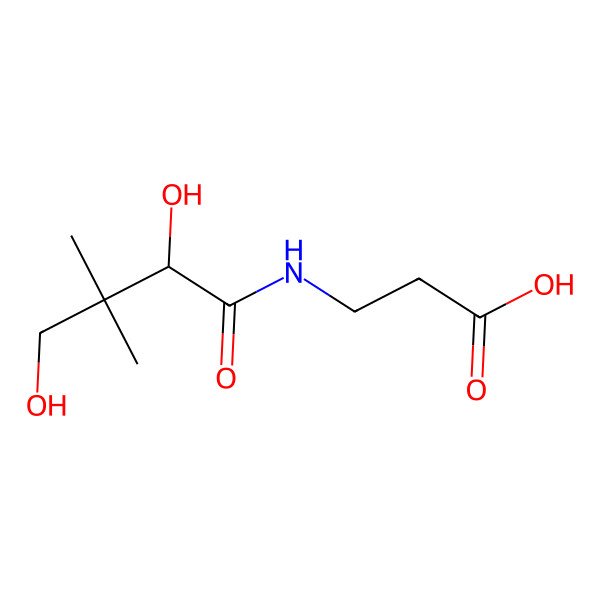
| D-pantothenic acid |
| vitamin B5 |
| 79-83-4 |
| pantothenate |
| Chick antidermatitis factor |
| (R)-pantothenate |
| (+)-Pantothenic acid |
| (R)-pantothenic acid |
| (D)-(+)-Pantothenic acid |
| HSDB 1020 |
| Kyselina pantothenova |
| Kyselina pantothenova [Czech] |
| Pantothenic acid (d) |
| PANTOTHENOIC ACID |
| BRN 1727064 |
| Pantothenic Acid [BAN] |
| D(+)-N-(2,4-Dihydroxy-3,3-dimethylbutyryl)-beta-alanine |
| beta-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-, (R)- |
| EINECS 201-229-0 |
| Vitamin B-5 |
| UNII-19F5HK2737 |
| CHEBI:46905 |
| Pantothenic acid, D- |
| 3-[[(2R)-2,4-dihydroxy-3,3-dimethylbutanoyl]amino]propanoic acid |
| N-(2,4-Dihydroxy-3,3-dimethylbutyryl)-beta-alanine |
| (D,+)-N(alpha-gamma-Dihydroxy-beta,beta-dimethylbutyryl)-beta-alanine |
| calcium pantothenate |
| 19F5HK2737 |
| DTXSID9023417 |
| 4-04-00-02569 (Beilstein Handbook Reference) |
| Pantothenic acid (BAN) |
| (+)-Pantothenate |
| N-[(2R)-2,4-DIHYDROXY-3,3-DIMETHYLBUTANOYL]-BETA-ALANINE |
| PANTOTHENIC ACID (MART.) |
| PANTOTHENIC ACID [MART.] |
| (R)-N-(2,4-Dihydroxy-3,3-dimethyl-1-oxobutyl)-beta-alanine |
| 3-[(2R)-2,4-dihydroxy-3,3-dimethylbutanamido]propanoic acid |
| N-[(2R)-2,4-Dihydroxy-3,3-dimethyl-1-oxobutyl]-beta-alanine |
| .beta.-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-, (R)- |
| d-pantothenate |
| PAU |
| B5, Vitamin |
| Vitamin B 5 |
| B 5, Vitamin |
| pantothenic-acid |
| Pantotenico acido |
| N-((2R)-2,4-DIHYDROXY-3,3-DIMETHYL-1-OXOBUTYL)-beta-ALANINE |
| delta-Pantothenate |
| D- pantothenic acid |
| Pantothen Pharmaselect |
| delta-Pantothenic acid |
| (R)-3-(2,4-Dihydroxy-3,3-dimethylbutanamido)propanoic acid |
| D-(+)-pantothenic acid |
| (D)-(+)-Pantothenate |
| bmse000287 |
| D07SJT |
| 'PANTOTHENOIC ACID' |
| SCHEMBL5436 |
| CHEMBL1594 |
| Pantothen Pharmaselect (TN) |
| VITAMIN B5 [VANDF] |
| PANTOTHENIC ACID [MI] |
| Pantothenic acid, D- (8CI) |
| DTXCID203417 |
| GTPL4668 |
| PANTOTHENIC ACID [HSDB] |
| PANTOTHENIC ACID [INCI] |
| PANTOTHENIC ACID [VANDF] |
| ()-pantothenate;()-vitamin B5 |
| VITAMIN B5 [GREEN BOOK] |
| GHOKWGTUZJEAQD-ZETCQYMHSA-N |
| PANTOTHENIC ACID [WHO-DD] |
| HMS2090P08 |
| HMS3656K08 |
| HY-B0430 |
| PANTOTHENIC ACID [ORANGE BOOK] |
| DB01783 |
| NCGC00346610-01 |
| AS-75446 |
| SBI-0206933.P001 |
| LS-101244 |
| SW220291-1 |
| C00864 |
| D07413 |
| E83797 |
| EN300-113766 |
| Q63390527 |
| D(+)-N-(2,4-Dihydroxy-3,3-dimethylbutyryl)-b-alanine |
| 450B5472-A689-4BCA-9BC1-58691B72D00F |
| N-[(2R)-2,4-Dihydroxy-3,3-dimethyl-1-oxobutyl]-ss-alanine |
| .beta.-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,(R)- |
| .beta.-Alanine, N-[(2R)-2,4-dihydroxy-3,3-dimethyl-1-oxobutyl]- |
| 3-((R)-2,4-Dihydroxy-3,3-dimethyl-butyrylamino)-propionic acid |
| 3-[[(2R)-2,4-dihydroxy-3,3-dimethyl-butanoyl]amino]propionic acid |
| Beta-alanina, n-[(2r)-2,4-dihidroxi-3],3-dimetil-1-oxobutil- |
| beta-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,(R)- |
| beta-Alanine, N-[(2R)-2,4-dihydroxy-3,3-dimethyl-1-oxobutyl]- |
| 'N-[(2R)-2,4-DIHYDROXY-3,3-DIMETHYLBUTANOYL]-BETA-ALANINE' |
| 3-[[(2R)-3,3-dimethyl-2,4-bis(oxidanyl)butanoyl]amino]propanoic acid |
| b-Alanine, N-[(2R)-2,4-dihydroxy-3,3-dimethyl-1-oxobutyl]- (9CI) |
| beta-Alanine, N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-, (R)- (9CI) |
| N-((2R)-2,4-DIHYDROXY-3,3-DIMETHYL-1-OXOBUTYL)-.BETA.-ALANINE |
|
There are more than 10 synonyms. If you wish to see them all click here.
|