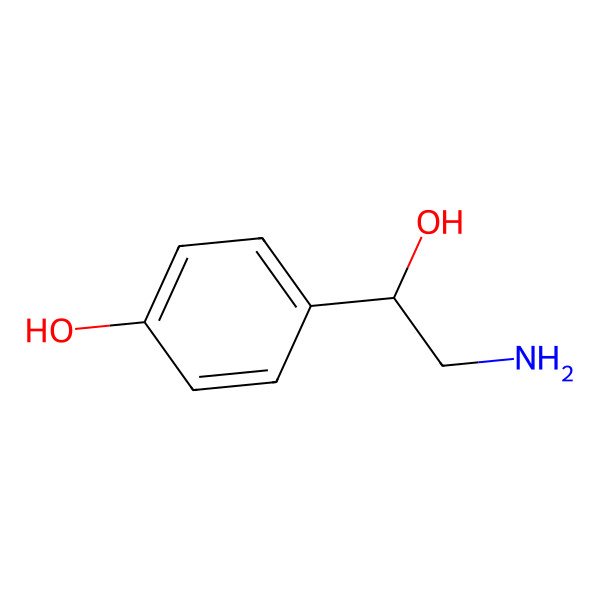
| Norsynephrine |
| 104-14-3 |
| 4-(2-Amino-1-hydroxyethyl)phenol |
| Norsympatol |
| Norsympathol |
| Norden |
| Norphen |
| Analet |
| octopaminum |
| Octopamine [INN] |
| Octapamine |
| Octopamina |
| Norfen |
| p-Norsynephrin |
| ND 50 |
| Paraoxyphenyl aminoethanol |
| Octopaminum [Latin] |
| Octopamina [Spanish] |
| para-Octopamine |
| 4-Octopamine |
| 1-(p-Hydroxyphenyl)-2-aminoethanol |
| Octopaminum [INN-Latin] |
| Octopamina [INN-Spanish] |
| WIN 5512 |
| p-Octopamine |
| p-Hydroxyphenylethanolamine |
| Octopamin |
| alpha-(Aminoethyl)-4-hydroxybenzenemethanol |
| alpha-(Aminomethyl)-p-hydroxybenzyl alcohol |
| beta-hydroxytyramine |
| WV 569 |
| alpha-Aminoethyl-4-hydroxybenzylalkohol |
| alpha-(Aminoethyl)-p-hydroxybenzyl alcohol |
| alpha-(aminomethyl)-4-hydroxybenzenemethanol |
| EINECS 203-179-5 |
| Benzenemethanol, alpha-(aminomethyl)-4-hydroxy- |
| BRN 1211019 |
| UNII-14O50WS8JD |
| dl-Octopamine |
| 14O50WS8JD |
| DTXSID7043873 |
| CHEBI:17134 |
| ND-50 |
| 4-(2-Amino-1-hydroxy-ethyl)phenol |
| NSC-757399 |
| NCGC00015760-06 |
| .alpha.-(Aminomethyl)-p-hydroxybenzyl alcohol |
| Benzyl alcohol, alpha-(aminomethyl)-p-hydroxy- |
| 4-13-00-02656 (Beilstein Handbook Reference) |
| DTXCID5023873 |
| Racemic octopamine |
| CAS-104-14-3 |
| octopamin- |
| 2-Amino-1-(4-hydroxyphenyl)ethanol |
| Spectrum_000201 |
| OCTOPAMINE [MI] |
| Prestwick0_000949 |
| Prestwick1_000949 |
| Prestwick2_000949 |
| Prestwick3_000949 |
| Spectrum2_000222 |
| Spectrum3_000275 |
| Spectrum4_000402 |
| Spectrum5_001543 |
| (.+/-.)-Octopamine |
| OCTOPAMINE [INCI] |
| D0L9YS |
| OCTOPAMINE [MART.] |
| OCTOPAMINE [WHO-DD] |
| Lopac0_000932 |
| SCHEMBL22605 |
| BSPBio_000918 |
| BSPBio_001750 |
| KBioGR_000664 |
| KBioSS_000681 |
| CHEMBL53929 |
| DivK1c_000245 |
| SPBio_000263 |
| SPBio_003077 |
| BPBio1_001010 |
| cid_102484 |
| GTPL2149 |
| BDBM32764 |
| KBio1_000245 |
| KBio2_000681 |
| KBio2_003249 |
| KBio2_005817 |
| KBio3_001250 |
| (A+/-)-Octopamine hydrochloride |
| NINDS_000245 |
| Tox21_110215 |
| BBL009983 |
| PDSP1_001544 |
| PDSP2_001528 |
| STK801377 |
| TBB066518 |
| 4-(2-Amino-1-hydroxyethyl)phenol # |
| AKOS000123349 |
| Tox21_110215_1 |
| CCG-205013 |
| DB13251 |
| NSC 757399 |
| SDCCGMLS-0066599.P001 |
| SDCCGSBI-0050906.P004 |
| IDI1_000245 |
| SMP1_000221 |
| 4-[(rs)-2-amino-1-hydroxyethyl]phenol |
| NCGC00015760-03 |
| NCGC00015760-05 |
| NCGC00015760-07 |
| NCGC00015760-08 |
| NCGC00015760-09 |
| NCGC00015760-10 |
| NCGC00015760-12 |
| NCGC00015760-14 |
| NCGC00015760-24 |
| NCGC00091918-03 |
| NCGC00091918-04 |
| LS-42692 |
| 4-[(1rs)-2-amino-1-hydroxyethyl]phenol |
| 4-[(IRS)-2-amino-1-hydroxyethyl]phenol |
| SBI-0050906.P003 |
| AB00053571 |
| 4-(2-amino-1-hydroxy-ethyl)phenol;hydrochloride |
| AB00053571-13 |
| AB00053571_14 |
| AB00053571_15 |
| EN300-1264124 |
| 4-(2-azanyl-1-oxidanyl-ethyl)phenol;hydrochloride |
| Benzenemethanol, .alpha.-(aminomethyl)-4-hydroxy- |
| Benzyl alcohol, .alpha.-(aminomethyl)-p-hydroxy- |
| Q424979 |
| 3,5-DIBROMOTYROSINETRIFLUOROACETAMIDEMETHYLESTER |
| (.+/-.)-.alpha.-(Aminomethyl)-p-hydroxybenzyl alcohol |
| 64C59BC0-9C98-49EF-A853-8849F02ABAAC |
| F2173-0210 |
| RACTOPAMINE HYDROCHLORIDE SUSPENSION IMPURITY, OCTOPAMINE- [USP IMPURITY] |
|
There are more than 10 synonyms. If you wish to see them all click here.
|