| 82-02-0 |
| Visammin |
| Methafrone |
| Amicardine |
| Viscardan |
| Coronin |
| Ammivisnagen |
| Amikellin |
| Ammipuran |
| Benecardin |
| Corafurone |
| Gynokhellan |
| Kelourin |
| Khelloyd |
| Medekellin |
| Rykellin |
| Ammivin |
| Ammispasmin |
| Interkellin |
| Interkhellin |
| Kelicorin |
| Khelangin |
| Khellamine |
| Khellanals |
| Khellinorm |
| Visammimix |
| Visnagalin |
| Kalangin |
| Kelamin |
| Kelincor |
| Norkel |
| Simeskellina |
| Kellosal |
| Khelfren |
| Khelisem |
| Lynamine |
| Mefurina |
| Vasokellina |
| Visnagen |
| Amiptan |
| Chellin |
| Kelicor |
| Kellin |
| Kellina |
| Keloid |
| Eskel |
| Cardio-khellin |
| Ammi-khellin |
| Bi-Kellina |
| Benekardin |
| Khelline I |
| Chellina |
| Khelline |
| Khellinum |
| Quelina |
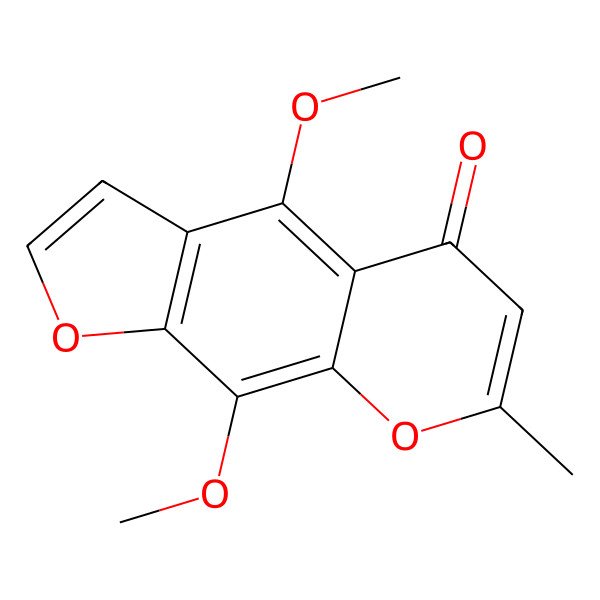
| 4,9-Dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-one |
| Ammicardine |
| 5,8-Dimethoxy-2-methyl-6,7-furanochromone |
| 4,9-Dimethoxy-7-methyl-5H-furo(3,2-g)(1)benzopyran-5-one |
| 4,9-Dimethoxy-7-methyl-5-oxofuro(3,2-g)-1,2-chromene |
| 5,8-Dimethoxy-2-methyl-4',5'-furo-6,7-chromone |
| IT-033 |
| CHEBI:6133 |
| Viscardin |
| 5H-Furo[3,2-g][1]benzopyran-5-one, 4,9-dimethoxy-7-methyl- |
| 5,8-Dimethoxy-2-methyl-4',5'-furano-6,7-chromone |
| EINECS 201-392-8 |
| UNII-5G117T0TJZ |
| NSC 25509 |
| NSC 37744 |
| NSC-25509 |
| NSC-37744 |
| 4,9-Dimethoxy-7-methyl-5-oxo-1,8-dioxabenz-(f)indene |
| 4,9-Dimethoxy-7-methyl-5-oxofuro(3,2-g)(1)benzopyran |
| BRN 0263185 |
| 5G117T0TJZ |
| 5H-Furo(3,2-g)(1)benzopyran-5-one, 4,9-dimethoxy-7-methyl- |
| DTXSID9045267 |
| AI3-52114 |
| NSC37744 |
| MFCD00005007 |
| 4,9-dimethoxy-7-methylfuro[3,2-g]chromen-5-one |
| Chorafurone |
| Vismagen |
| Khell |
| MLS000028448 |
| DTXCID7025267 |
| 4,9-Dimethoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one |
| NSC8519 |
| 5-19-06-00320 (Beilstein Handbook Reference) |
| 5,7-furanochromone |
| CAS-82-02-0 |
| NCGC00016327-01 |
| SMR000058278 |
| 4,2-g][1]benzopyran |
| KHELLIN (MART.) |
| KHELLIN [MART.] |
| 4,8-dioxabenz-[f]indene |
| 4,2-g]-1,2-chromene |
| 5,5'-furo-6,7-chromone |
| 5,5'-furano-6,7-chromone |
| Chellina [Italian] |
| 4,2-g][1]benzopyran-5-one |
| Khellin [INN:DCF] |
| Khelline [INN-French] |
| Khellinum [INN-Latin] |
| Quelina [INN-Spanish] |
| 5H-Furo[3, 4,9-dimethoxy-7-methyl- |
| WLN: T C566 DO JV MOJ BO1 HO1 L1 |
| SR-01000000072 |
| 4,9-dimethoxy-7-methyl-5H-furo(3,2-g)chromen-5-one |
| kelina |
| Gynokhellin |
| Intercellin |
| Hkelfren |
| Prestwick_287 |
| Spectrum_000079 |
| Khellin, for microscopy |
| Opera_ID_372 |
| 4,9-Dimethoxy-7-methyl-5H-furo[3,2-g]-[1]benzopyran-5-one |
| KHELLIN [INN] |
| KHELLIN [MI] |
| Prestwick0_000091 |
| Prestwick1_000091 |
| Prestwick2_000091 |
| Prestwick3_000091 |
| Spectrum2_000593 |
| Spectrum3_000654 |
| Spectrum4_001557 |
| Spectrum5_000154 |
| KHELLIN [WHO-DD] |
| bmse000751 |
| SCHEMBL9655 |
| Khellin, analytical standard |
| BSPBio_000042 |
| BSPBio_002287 |
| KBioGR_002054 |
| KBioSS_000479 |
| SPECTRUM210866 |
| MLS001076533 |
| CHEMBL44746 |
| DivK1c_000046 |
| 5,9-dimethoxy-2-methylfurano[3,2-g]chromen-4-one |
| SPBio_000466 |
| SPBio_001981 |
| BPBio1_000048 |
| MEGxp0_000331 |
| ACon0_000983 |
| ACon1_000350 |
| HMS500C08 |
| HSMPDPBYAYSOBC-UHFFFAOYSA- |
| KBio1_000046 |
| KBio2_000479 |
| KBio2_003047 |
| KBio2_005615 |
| KBio3_001507 |
| NINDS_000046 |
| HMS1568C04 |
| HMS1923M07 |
| HMS2095C04 |
| HMS2230B16 |
| HMS3371C21 |
| HMS3712C04 |
| Pharmakon1600-00210866 |
| HY-B1394 |
| NSC-8519 |
| NSC25509 |
| Tox21_110374 |
| BDBM50480260 |
| CCG-36453 |
| LMPK13110001 |
| NSC755826 |
| s5887 |
| AKOS002281934 |
| Tox21_110374_1 |
| NSC-755826 |
| SDCCGMLS-0003040.P003 |
| IDI1_000046 |
| NCGC00016327-02 |
| NCGC00016327-03 |
| NCGC00016327-04 |
| NCGC00016327-05 |
| NCGC00016327-06 |
| NCGC00016327-07 |
| NCGC00016327-08 |
| NCGC00016327-09 |
| NCGC00016327-11 |
| NCGC00023424-03 |
| NCGC00023424-04 |
| NCGC00169160-01 |
| NCGC00169160-02 |
| NCGC00169160-03 |
| AS-35307 |
| SY051635 |
| SBI-0051567.P002 |
| CS-0013121 |
| FT-0627578 |
| K0039 |
| C09010 |
| K-3400 |
| AB00052134_16 |
| 4,9-Dimethoxy-7-methyl-furo[3,2-g]chromen-5-one |
| Q2079998 |
| SR-01000000072-3 |
| SR-01000000072-4 |
| SR-01000000072-5 |
| SR-01000000072-6 |
| BRD-K80353807-001-05-5 |
| BRD-K80353807-001-06-3 |
| BRD-K80353807-001-16-2 |
| 4,9-dimethoxy-7-methylpyrano[3,2-f][1]benzoxol-5-one |
| 4,9-Dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-one # |
| InChI=1/C14H12O5/c1-7-6-9(15)10-11(16-2)8-4-5-18-12(8)14(17-3)13(10)19-7/h4-6H,1-3H3 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|