| 33279-57-1 |
| k-Strofantozyd |
| k-Strophantosid |
| k-Strophantoside |
| CHEMBL538442 |
| UNII-8141H9654J |
| k-Strophanthidin-gamma |
| k-Strofantozyd [Polish] |
| k-Strophantosid [German] |
| 8141H9654J |
| Strophanthidin + cymarose + beta-glucose + alpha-glucose |
| NSC 7530 |
| EINECS 251-439-1 |
| k-Strophanthin-gamma |
| Card-20(22)-enolide, 3-[(O-beta-D-glucopyranosyl-(1-->6)-O-beta-D-glucopyranosyl-(1-->4)-2,6-dideoxy-3-O-methyl-beta-D-ribo-hexopyranosyl)oxy]-5,14-dihydroxy-19-oxo-, (3beta,5beta)- |
| STROPHANTHOSIDE-K |
| NSC7530 |
| NSC-7530 |
| C42H64O19 |
| Strophanthidin + cymarose + beta-glucose + alpha-glucose [German] |
| C42-H64-O19 |
| SCHEMBL3866065 |
| NIOSH/WL1200000 |
| DTXSID60954919 |
| (3beta,5beta)-3-((O-beta-D-glucopyranosyl-(1.6)-O-beta-D-glucopyranosyl-(1.4)-2,6-dideoxy-3-O-methyl-beta-D-ribo-hexopyranosyl)oxy)-5,14-dihydroxy-19-oxocard-20(22)-enolide |
| (3beta5beta)-3-[(O-beta-D-glucopyranosyl-(1.fwdarw.6)-O-beta-D-glucopyranosyl-(1.fwdarw.4)-2,6-dideoxy-3-O-methyl-beta-D-ribo-hexopyranosyl)oxy]-5,14-dihydroxy-19-oxocard-20(22)-enolide |
| 5-beta-Card-20(22)-enolide, 3-beta-((O-beta-D-glucopyranosyl-(1-6)-O-beta-D-glucopyranosyl-(1-4)-2,6-dideoxy-3-O-methyl-beta-D-ribo-hexopyranosyl)oxy)-5,14-dihydroxy-19-oxo- |
| Card-20(22)-enolide, 3-((O-beta-D-glucopyranosyl-(1-6)-O-beta-D-glucopyranosyl-(1-4)-2,6-dideoxy-3-O-methyl-beta-D-ribo-hexopyranosyl)oxy)-5,14-dihydroxy-19-oxo-, (3beta,5beta)- |
| Card-20(22)-enolide, 3-[(O-.beta.-D-glucopyranosyl-(1.fwdarw.6)-O-.beta.-D-glucopyranosyl-(1.fwdarw.4)-2,6-dideoxy-3-O-methyl-.beta.-D-ribo-hexopyranosyl)oxy]-5,14-dihydroxy-19-oxo-, (3.beta.,5.beta.)- |
| BDBM50480588 |
| AKOS040761932 |
| WL12000000 |
| k-Strophantoside (Strophanthidin-Cym-Glc-Glc) |
| 3-{[hexopyranosyl-(1->6)hexopyranosyl-(1->4)-2,6-dideoxy-3-o-methylhexopyranosyl]oxy}-5,14-dihydroxy-19-oxocard-20(22)-enolide |
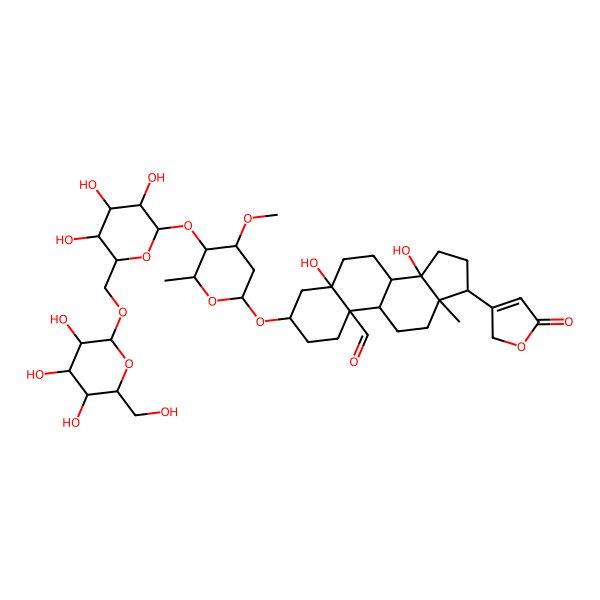
| 5,14-dihydroxy-3-[4-methoxy-6-methyl-5-[3,4,5-trihydroxy-6-[[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-tetrahydropyran-2-yl]oxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-10-carbaldehyde |
| CARD-20(22)-ENOLIDE, 3-((O-.BETA.-D-GLUCOPYRANOSYL-(1->6)-O-.BETA.-D-GLUCOPYRANOSYL-(1->4)-2,6-DIDEOXY-3-O-METHYL-.BETA.-D-RIBO-HEXOPYRANOSYL)OXY)-5,14-DIHYDROXY-19-OXO-, (3.BETA.,5.BETA.)- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|