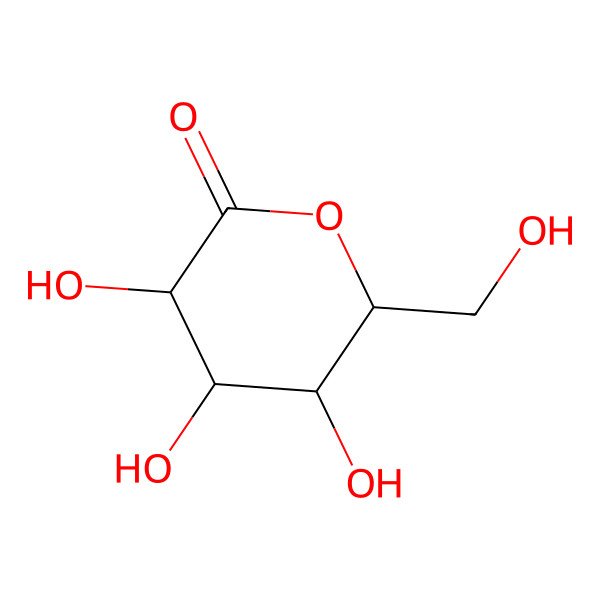
| delta-gluconolactone |
| 90-80-2 |
| D-glucono-1,5-lactone |
| Gluconic acid lactone |
| 1,5-Gluconolactone |
| Glucono delta-lactone |
| D-Gluconolactone |
| d-(+)-Glucono-1,5-lactone |
| D-Gluconic acid lactone |
| D-Gluconic acid delta-lactone |
| 1,5-D-Gluconolactone |
| Gluconic lactone |
| Glucono delta lactone |
| Gluconic delta-lactone |
| (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-one |
| D-delta-Gluconolactone |
| delta-D-Gluconolactone |
| Deltagluconolactone |
| Fujiglucon |
| D-Gluconic delta-lactone |
| glucono-delta-lactone |
| (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-one |
| Glucarolactone |
| beta-Glucono-1,5-lactone |
| Lysactone |
| D-Aldonolactone |
| Riken lactone |
| D-(+)-Gluconic acid delta-lactone |
| D-Gluconic acid-delta-lactone |
| Gluconolactone [USP] |
| HSDB 488 |
| UNII-WQ29KQ9POT |
| WQ29KQ9POT |
| AI3-19578 |
| EINECS 202-016-5 |
| D-threo-Aldono-1,5-lactone |
| INS NO.575 |
| 1335-57-5 |
| DTXSID0026549 |
| glucono-1,5-lactone |
| CHEBI:16217 |
| INS-575 |
| D-Gluconic acid, delta-lactone |
| Gluconic Acid Anhydride |
| Gluconic acid, delta-lactone, D- |
| Glucono .delta. lactone |
| Glucono .delta.-lactone |
| .delta.-D-Gluconolactone |
| NSC 34393 |
| NSC-34393 |
| 4253-68-3 |
| NSC-758238 |
| Gluconic Acid delta-Lactone |
| GLUCONO-DELTA LACTONE |
| Gluconic acid lactone (6CI) |
| DTXCID406549 |
| E-575 |
| d-Gluconic acid .delta.-lactone |
| EC 202-016-5 |
| Gluconolactone (USP) |
| RENACIDIN COMPONENT GLUCONOLACTONE |
| D-glucono-delta-lactone |
| GLUCONOLACTONE COMPONENT OF RENACIDIN |
| (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-one |
| GLUCONOLACTONE (II) |
| GLUCONOLACTONE [II] |
| 135820-79-0 |
| GDL |
| GLUCONOLACTONE (MART.) |
| GLUCONOLACTONE [MART.] |
| GLUCONOLACTONE (USP-RS) |
| GLUCONOLACTONE [USP-RS] |
| 3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-one |
| D-Gluconic acid, .delta.-lactone |
| Glucolactone |
| d-gluconic acid d-lactone |
| GLUCONOLACTONE (USP MONOGRAPH) |
| GLUCONOLACTONE [USP MONOGRAPH] |
| CAS-90-80-2 |
| .delta.-Gluconolactone |
| LGC |
| D-Gluconic acid-1,5-lactone |
| gluconolactones |
| NSC34393 |
| Glucono--Lactone |
| gluconodeltalactone |
| delta-Aldonolactone |
| Gluconate, lactone |
| NCGC00095002-01 |
| delta gluconolactone |
| gamma-Gluconolactone |
| D-Glucono-d-lactone |
| MFCD00006647 |
| Glucono gamma-lactone |
| Glucono 1,5-lactone |
| D-glucon-1,5-lacton |
| D-glucono1,5-lactone |
| gluconic acid d-lactone |
| delta-delta-Gluconolactone |
| 1,5-delta-Gluconolactone |
| bmse000230 |
| D04QWD |
| delta-Gluconic acid lactone |
| delta-Glucono-delta-lactone |
| GLUCONOLACTONE [MI] |
| delta-Glucono-1,5-lactone |
| delta-Gluconic delta-lactone |
| Glucono-Delta-Lactone(GDL) |
| SCHEMBL15320 |
| GLUCONOLACTONE [HSDB] |
| GLUCONOLACTONE [INCI] |
| delta-Gluconic acid d-lactone |
| MLS002207105 |
| D-Gluconic acid 1,5-lactone |
| D- glucono- 1, 5- lactone |
| GLUCONOLACTONE [WHO-DD] |
| CHEMBL1200829 |
| D-(+)-Gluconic acid d-lactone |
| CHEBI:24267 |
| D-Gluconic acid, <V-lactone |
| delta-Gluconic acid 1,5-lactone |
| delta-Gluconic acid delta-lactone |
| delta-Gluconic acid-1,5-lactone |
| delta-Gluconic acid-delta-lactone |
| PHOQVHQSTUBQQK-SQOUGZDYSA-N |
| D(+)-Gluconic acid gamma-lactone |
| Glucono-|A-lactone USP26 FCCIV |
| CS-M3768 |
| D-(+)-Gluconic acid-delta lactone |
| delta-(+)-Gluconic acid d-lactone |
| GLUCONO DELTA-LACTONE [FCC] |
| HY-I0301 |
| GLUCONOLACTONE [ORANGE BOOK] |
| Tox21_111383 |
| Tox21_200429 |
| BDBM50366565 |
| GLUCONO-DELTA-LACTONE [VANDF] |
| AKOS016843888 |
| Tox21_111383_1 |
| DB04564 |
| delta-(+)-Gluconic acid-delta lactone |
| DS-4779 |
| LS-2264 |
| (3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-one |
| NCGC00257983-01 |
| NCGC00344522-01 |
| AC-13150 |
| E575 |
| SMR001306715 |
| G0039 |
| EN300-97037 |
| C00198 |
| D04332 |
| P19765 |
| D-(+)-Gluconic acid delta-lactone, >=99.0% |
| Gluconolactone, meets USP testing specifications |
| Q114174 |
| W-100325 |
| 3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-one |
| D-(+)-Gluconic acid delta-lactone, analytical standard |
| Z1255427181 |
| A88519CB-A562-4C9C-B925-0A6B1701F841 |
| Gluconolactone, United States Pharmacopeia (USP) Reference Standard |
| (3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydropyran-2-one |
| (3S,4R,5R,6S)-3,4,5-TRIHYDROXY-6-(HYDROXYMETHYL)TETRAHYDRO-2H-PYRAN-2-ONE; GLUCONOLACTONE |
| D-GLUCONIC ACID DELTA-LACTONE(O'Neil, M.J. (ed.). The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 793) |
| GLUCONO DELTA LACTONE(O'Neil, M.J. (ed.). The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 793) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|