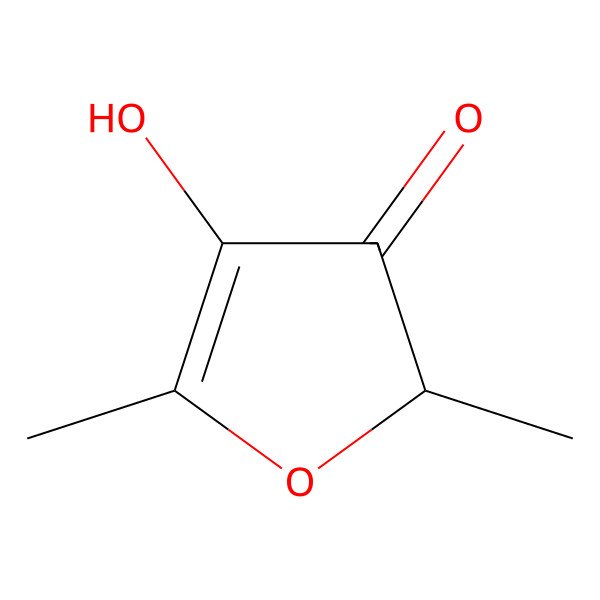
| 3658-77-3 |
| 4-hydroxy-2,5-dimethylfuran-3(2H)-one |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone |
| 4-Hydroxy-2,5-dimethyl-3(2H)furanone |
| 2,5-Dimethyl-4-hydroxy-3(2H)-furanone |
| Pineapple ketone |
| Dimethylhydroxy furanone |
| Alletone |
| 3(2H)-Furanone, 4-hydroxy-2,5-dimethyl- |
| 4-hydroxy-2,5-dimethylfuran-3-one |
| Strawberry furanone |
| 4-hydroxy-2,5-dimethyl-3-furanone |
| Coe 536 |
| FEMA No. 3174 |
| FEMA 3174 |
| 2,5-Dimethyl-4-hydroxy-2,3-dihydrofuran-3-one |
| 2,5-Dimethyl-3-hydroxy-4-oxo-4,5-dihydrofuran |
| 4-Hydroxy-2,5-dimethylfuran-2(3H)-one |
| EINECS 222-908-8 |
| 4-hydroxy-2,5-dimethyl-2,3-dihydrofuran-3-one |
| UNII-20PI8YZP7A |
| 3(2H)-FURANONE, 2,5-DIMETHYL-4-HYDROXY- |
| BRN 1281357 |
| 20PI8YZP7A |
| CCRIS 8102 |
| DTXSID0041517 |
| CHEBI:76247 |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (natural) |
| MFCD00010706 |
| 192466-95-8 |
| 2,5-Dimethyl-4-hydroxy-3(2H)-furanone-13C2 |
| Furaneol Natural |
| Nat. Furaneol |
| Furaneol,Synthetic |
| ALNOSE |
| HDMF |
| dimethylhydroxyfuranone |
| (+/-)-Furaneol |
| SCHEMBL57008 |
| CHEMBL3186302 |
| DIMETHYL DIHYDROFURANOLONE |
| DTXCID8021517 |
| HSDB 8322 |
| HY-N7093 |
| Tox21_300823 |
| 2,5-DIMETHYLDIHYDROFURANOLONE |
| AC8010 |
| s5178 |
| AKOS015900499 |
| CS-W013470 |
| DIMETHYLHYDROXY FURANONE [INCI] |
| LS-2407 |
| SB60884 |
| NCGC00248185-01 |
| NCGC00254727-01 |
| 2,5-dimethyl-4-hydroxy-3[2H] furanone |
| 4-hydroxy-2,5-dimethyl-3-oxo-2H-furan |
| AC-14027 |
| AS-15673 |
| SY035594 |
| CAS-3658-77-3 |
| 2,5-dimethyl-4-hydroxy-3-(2H)-furanone |
| 2.5-dimethyl-4-hydroxy-3-(2H)-furanone |
| 3(2h)-furanona, 4-hidroxi-2,5-dimetil- |
| 4-hydroxy-2,5-dimethyl-furan-3(2H)-one |
| 2,5-dimethyl-4-hydroxy-(2h)-furan-3-one |
| 2,5-dimethyl-4-hydroxy-3-oxo-(2H)-furan |
| D1569 |
| D1884 |
| FT-0610446 |
| C20717 |
| EN300-175904 |
| 2,3-Dihydro-4-hydroxy-2,5-dimethyl-3-furanone |
| 4-oxo-3-hydroxy-2,5-dimethyl-4,5-dihydrofuran |
| 4- hydroxy- 2, 5- dimethylfuran- 2(3H)- one |
| A823300 |
| Q250455 |
| Q-100434 |
| 4 - hydroxy - 2,5 - dimethylfuran - 2(3H) - one |
| 4-HYDROXY-2,5-DIMETHYL-3(2H)-FURANONE [FCC] |
| 4-HYDROXY-2,5-DIMETHYL-3(2H)-FURANONE [FHFI] |
| Z1255395050 |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, >=99.0% (GC) |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, >=98%, FCC, FG |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, analytical standard |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, natural, >=98%, FG |
| InChI=1/C6H8O3/c1-3-5(7)6(8)4(2)9-3/h3,8H,1-2H |
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone, 15 wt. % (in propylene glycol), FG |
|
There are more than 10 synonyms. If you wish to see them all click here.
|