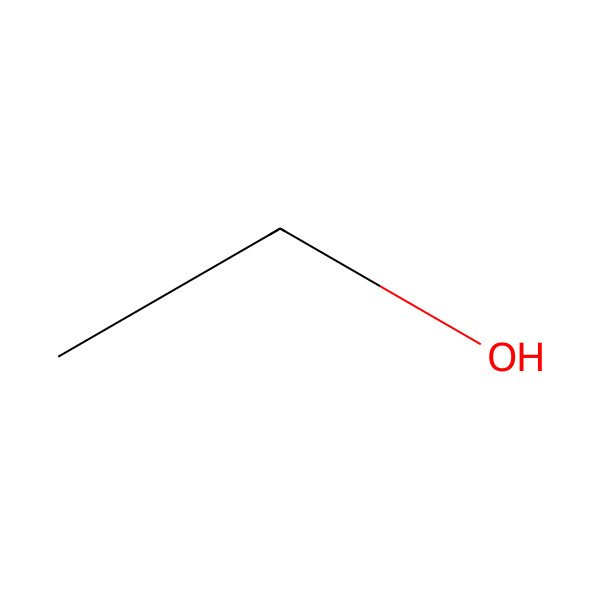
| ethyl alcohol |
| alcohol |
| 64-17-5 |
| grain alcohol |
| Methylcarbinol |
| Ethyl hydroxide |
| Ethyl hydrate |
| Tecsol |
| Algrain |
| Anhydrol |
| EtOH |
| Hydroxyethane |
| Alkohol |
| Jaysol S |
| Potato alcohol |
| 1-Hydroxyethane |
| Ethanol 200 proof |
| Aethylalkohol |
| Aethanol |
| Absolute ethanol |
| Alcool ethylique |
| etanol |
| Denatured alcohol |
| Denatured ethanol |
| Fermentation alcohol |
| Tecsol C |
| Dehydrated ethanol |
| Alcool etilico |
| Alcohol, ethyl |
| Alcohol, diluted |
| Etanolo |
| Jaysol |
| Etylowy alkohol |
| absolute alcohol |
| Alcohol dehydrated |
| Alkoholu etylowego |
| Dehydrated alcohol |
| Ethyl alcohol usp |
| Synasol |
| Alcohol, anhydrous |
| Ethyl alcohol anhydrous |
| Alcohol anhydrous |
| Ethylalcohol |
| Alcohol, dehydrated |
| Denatured alcohol CD-5 |
| Denatured alcohol SD-1 |
| SD Alchol 23-hydrogen |
| Denatured alcohol CD-5a |
| Denatured alcohol SD-3a |
| Denatured alcohol CD-10 |
| Denatured alcohol SD-17 |
| Denatured alcohol SD-28 |
| Denatured alcohol SD-30 |
| Denatured alcohol SD-13a |
| Denatured alcohol SD-23a |
| Denatured alcohol SD-39b |
| Denatured alcohol SD-39c |
| Denatured alcohol SD-40m |
| Spirt |
| Ethanol, undenatured |
| Alcare Hand Degermer |
| Ethanol Absolute |
| Ethyl alcohol & water, 5% |
| Ethyl alcohol & water, 10% |
| Ethyl alcohol & water, 20% |
| Ethyl alcohol & water, 30% |
| Ethyl alcohol & water, 40% |
| Ethyl alcohol & water, 50% |
| Ethyl alcohol & water, 60% |
| Ethyl alcohol & water, 70% |
| Ethyl alcohol & water, 80% |
| Ethyl alcohol & water, 95% |
| Ethyl alcohol & water, 96% |
| Alkohol [German] |
| Aethanol [German] |
| Etanolo [Italian] |
| Alcohol (ethyl alcohol) |
| Hinetoless |
| Ethicap |
| Thanol |
| NCI-C03134 |
| Desinfektol EL |
| Ethyl alc |
| Ethylalcohol [Dutch] |
| Anhydrous ethanol |
| Caswell No. 430 |
| Ethyl alcohol and water |
| Alcohol, Absolute |
| FEMA Number 2419 |
| Alcohol [USP] |
| Ethanol [JAN] |
| Ethyl alcohol, undenatured |
| C2H5OH |
| Ethylicum |
| Ethylol |
| alcohol etilico |
| Edible alcohol |
| Alcohol,ethyl |
| Reagent Alcohol |
| Infinity Pure |
| Aethylalkohol [German] |
| HSDB 82 |
| CCRIS 945 |
| Alcohol, Grain |
| Anhydrous alcohol |
| Ethanol Anhydrous |
| Ethyl alcohol in alcoholic beverages |
| Alcohol,dehydrated |
| FEMA No. 2419 |
| SD alcohol 23-hydrogen |
| AI3-01706 |
| Alcool etilico [Italian] |
| Etylowy alkohol [Polish] |
| Alcool ethylique [French] |
| Ethanol, dehydrated |
| SY Fresh M |
| Alkoholu etylowego [Polish] |
| Esumiru WK 88 |
| SDM No. 37 |
| NSC 85228 |
| Anhydrol PM 4085 |
| Sekundasprit |
| Alcoholum |
| Ethanolum |
| Ethyl alcohol, absolute |
| Ethyl alcohol, anhydrous |
| Ethyl alcohol (Ethanol) |
| Ethanol Vapor |
| Ethyl alcohol, dehydrated |
| Alcohol denatured |
| Alcohol (ethyl) |
| SDA 3A |
| Ru-Tuss Hydrocodone Liquid |
| ALCOHOL 5% IN DEXTROSE 5% |
| Alcohol, denatured |
| EINECS 200-578-6 |
| C2H6O |
| Ethanol, Anhydrous |
| Alcohol (USP) |
| Alcohol determination--alcohol |
| EPA Pesticide Chemical Code 001501 |
| ABLYSINOL |
| Ethanol (9CI) |
| 100C.NPA |
| IMS 99 |
| Absolute ethyl alcohol |
| DTXSID9020584 |
| UNII-3K9958V90M |
| CHEBI:16236 |
| ALCOHOL DENAT. |
| Punctilious ethyl alcohol |
| SDA 40-2 |
| 3K9958V90M |
| Ethanol-1-13C (9CI) |
| DTXCID30584 |
| Ethanol in alcoholic beverages |
| ALCOHOL 5% IN D5-W |
| CDA 19 |
| HBN-1 COMPONENT ETHANOL |
| AVAGARD COMPONENT ALCOHOL |
| UNII-7528N5H79B |
| PM-6193-200 |
| EC 200-578-6 |
| Ethanol, denatured |
| ETHANOL COMPONENT OF HBN-1 |
| AHD 2000 |
| ALCOHOL COMPONENT OF AVAGARD |
| NSC85228 |
| NSC-85228 |
| CDA 19-200 |
| Ethanol, CDA 19 |
| Ethanol, 200 Proof |
| Ethyl Alcohol Denatured |
| B3324 |
| EOH |
| Ethyloxy Group |
| C2-H6-O |
| MFCD00003568 |
| Ethanol denatured |
| Ethanol Extra Pure |
| 42845-45-4 |
| 68475-56-9 |
| 71076-86-3 |
| 8000-16-6 |
| 8024-45-1 |
| ETHANOL, ANHYDROUS (EP MONOGRAPH) |
| ETHANOL, ANHYDROUS [EP MONOGRAPH] |
| Lux |
| Oxydimethylene Group |
| Ethanol Absolute Bp |
| ETHANOL IN ALCOHOLIC BEVERAGES (IARC) |
| ETHANOL IN ALCOHOLIC BEVERAGES [IARC] |
| 68476-78-8 |
| HYDROXYETHYL GROUP |
| Ethyl Alcohol, Denatured |
| Athylalkohol |
| Athanol |
| methyl carbinol |
| diluted Alcohol |
| Vodka |
| Alcohol,sda |
| ethanol- |
| Sd alcohol |
| Alcohol,denatured |
| undenatured Ethanol |
| Ethanolum anhydricum |
| QMHAIh |
| VANILLA Powder |
| Alcohol [USAN] |
| Eosin Y, Alcoholic |
| Alcohol 95% |
| fermentation alcohol |
| Alcohol 190 proof |
| Ethyl Alcohol 40% |
| Ethyl Alcohol 70% |
| Ethyl Alcohol 75% |
| Ethyl Alcohol 80% |
| Ethyl Alcohol 90% |
| EAL (CHRIS Code) |
| ALCOHOL [VANDF] |
| ALCOHOL [HSDB] |
| ALCOHOL [INCI] |
| Ethanol, 99.8% |
| Ethanol, technical grade |
| Reagent Alcohol, 70% |
| Reagent Alcohol, 80% |
| Reagent Alcohol, 95% |
| (OEtH) |
| ALCOHOL [II] |
| CH3CH2OH |
| Dehydrated ethanol (TN) |
| Ethyl Alcohol 47.5% |
| ETHYLICUM [HPUS] |
| Ethanol, standard for GC |
| Ethyl alcohol; (ethanol) |
| ALCOHOL [USP-RS] |
| ALCOHOL [WHO-IP] |
| ETHANOL [WHO-DD] |
| ETHANOL [WHO-IP] |
| bmse000297 |
| CHEMBL545 |
| D00AMQ |
| ETHANOL (ANHYDROUS) |
| Ethanol, >=99.5% |
| Anhydrous ethanol (JP17) |
| ETHYL ALCOHOL [MI] |
| Reagent Alcohol, for HPLC |
| E7148_ALDRICH |
| Ethanol, analytical standard |
| (CH2Me(OH)) |
| Ethyl alcohol (DOT:OSHA) |
| SD 3A |
| Ru-Tuss Liquid (Salt/Mix) |
| Ethanol, USP, 99.5% |
| ETHYL ALCOHOL [FHFI] |
| WLN: Q2 |
| Ethanol, anhydrous, denatured |
| Reagent Alcohol, ACS reagent |
| ALCOHOL [ORANGE BOOK] |
| ALCOHOL,ETHYL [VANDF] |
| B3324 [LANGUAL] |
| Ethanol, p.a., 99.8% |
| Reagent Alcohol, reagent grade |
| SDM No. 37 (Salt/Mix) |
| GTPL2299 |
| ANHYDROUS ETHANOL [JAN] |
| Ethanol, denatured (5% IPA) |
| Alcohol dehydrated, >=85.0% |
| Ethanol, technical grade, 93% |
| Ethanol, technical grade, 99% |
| CHEBI:17246 |
| Ethanol, 95.1-96.9% |
| Ru-Tuss Expectorant (Salt/Mix) |
| ALCOHOL, DEHYDRATED [II] |
| ALCOHOLUM [WHO-IP LATIN] |
| ETHANOLUM [WHO-IP LATIN] |
| Ethyl alcohol (6CI,7CI,8CI) |
| ALCOHOL,DEHYDRATED [VANDF] |
| LTBB002977 |
| Ethanol, technical grade, 93.8% |
| Ethanol, technical grade, 99.5% |
| ALCOHOL, DEHYDRATED [VANDF] |
| DEHYDRATED ALCOHOL [USP-RS] |
| Ethanol, >=99.5%, for HPLC |
| STR05604 |
| EINECS 270-649-4 |
| Ethyl Alcohol 95% ACS/USP Grade |
| Tox21_202510 |
| ALCOHOL 5% AND DEXTROSE 5% |
| NA1170 |
| STL264245 |
| UN1170 |
| Ethanol 2000 microg/mL in Methanol |
| Ethanol, 95.0%, (190 proof) |
| Ethanol, p.a., ACS reagent, 96% |
| Ethanol, tested according to Ph.Eur. |
| Ethanol, USP, 70.0-72.0% |
| Ethanol, USP, 94.9-96.0% |
| AKOS009104571 |
| Ethanol solutions (UN 1170)(DOT) |
| Ethanol, Reagent (Denatured SDA 3A) |
| 7528N5H79B |
| ALCOHOL 10% AND DEXTROSE 5% |
| DB00898 |
| Ethanol 10000 microg/mL in Methanol |
| Ethanol, absolute, >=99.8% (GC) |
| Ethanol, UV HPLC spectroscopic, 95% |
| LS-1539 |
| UN 1170 |
| CAS-64-17-5 |
| Ethanol, denatured, (UK IDA standard) |
| Ethanol, SAJ first grade, >=99.5% |
| Ethyl Alcohol Absolute, ACS/USP Grade |
| USEPA/OPP Pesticide Code: 001501 |
| Ethanol, JIS special grade, >=99.5% |
| Ethanol, p.a., ACS reagent, 95.0% |
| Ethanol, technical grade, 92.6-93.8 |
| NCGC00091458-01 |
| NCGC00091458-02 |
| NCGC00260059-01 |
| Reagent Alcohol, spectrophotometric grade |
| DEHYDRATED ALCOHOL [USP MONOGRAPH] |
| EOX |
| Q153 |
| Ethanol, absolute, for HPLC, >=99.8% |
| Ethyl Alcohol 95% (Synthetic) FCC Grade |
| Ethanol, ACS reagent, 99.8, 200 proof |
| Ethanol, JIS first grade, 94.8-95.8% |
| Ethyl Alcohol Absolute (Organic) USP Grade |
| E1510 |
| Ethanol, denatured, Spectrophotometric Grade |
| Ethanol, JIS special grade, 94.8-95.8% |
| FT-0625729 |
| FT-0625731 |
| FT-0625732 |
| FT-0668048 |
| Ethanol, >=99.5%, SAJ super special grade |
| Reagent Alcohol, anhydrous, <=0.003% water |
| Reagent Alcohol, anhydrous, <=0.005% water |
| C00469 |
| D00068 |
| Ethanol, >=99.5%, suitable for fluorescence |
| Ethanol, Alcohol Reagent, anhydrous, denatured |
| Ethyl Alcohol 95% (Grain Derived) FCC Grade |
| Ethyl Alcohol 95% (Synthetic) ACS/USP Grade |
| InChI=1/C2H6O/c1-2-3/h3H,2H2,1H |
| Ethyl Alcohol Absolute (Synthetic) ACS/USP Grade |
| SR-01000944357 |
| Ethanol, puriss. p.a., absolute, >=99.8% (GC) |
| SR-01000944357-1 |
| Ethanol, denatured (5 % IPA, 5 % n-propylacetate) |
| Reagent Alcohol, used for histology tissue preparation |
| Ethyl alcohol, Pure, 190 proof, for molecular biology |
| Ethyl alcohol, Pure, 200 proof, anhydrous, >=99.5% |
| Ethyl alcohol, Pure, 200 proof, for molecular biology |
| Ethanol 70%, denatured with 1% MEK, 1% IPA, 10 mg/L |
| Ethanol, absolute, >=99.8% (GC), sales not in Germany |
| Ethyl Alcohol Absolute (Dehydrated) USP, BP/EP, JP Grade |
| Ethyl alcohol, Pure, 200 proof, ACS reagent, >=99.5% |
| Reagent Alcohol, 70%, used for histology tissue preparation |
| Reagent Alcohol, 80%, used for histology tissue preparation |
| 1E37B0D2-6209-4B03-A57D-500F3223C2DA |
| Alcohol, United States Pharmacopeia (USP) Reference Standard |
| Ethanol, >=99.5%, suitable for absorption spectrum analysis |
| Ethyl alcohol, Pure, 200 proof, HPLC/spectrophotometric grade |
| Reagent Alcohol, 95%, Used for histology tissue preparation |
| Ethanol, p.a., ACS reagent, reag. ISO, reag. Ph. Eur., 99.9% |
| Ethyl alcohol, Pure, 190 proof, meets USP testing specifications |
| Ethyl alcohol, Pure, 200 proof, anhydrous, ZerO2(TM), >=99.5% |
| Ethyl alcohol, Pure, 200 proof, meets USP testing specifications |
| Specially Denatured Alcohol, 190 proof, SDA 23A, contains Acetone |
| Specially Denatured Alcohol, 190 proof, SDA 2B-3, contains Toluene |
| Specially Denatured Alcohol, 190 proof, SDA 30, contains Methanol |
| Specially Denatured Alcohol, 190 proof, SDA 3A, contains Methanol |
| Specially Denatured Alcohol, 200 proof, SDA 23A, contains Acetone |
| Specially Denatured Alcohol, 200 proof, SDA 2B-3, contains Toluene |
| Specially Denatured Alcohol, 200 proof, SDA 30, contains Methanol |
| Specially Denatured Alcohol, 200 proof, SDA 3A, contains Methanol |
| Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material |
| Dehydrated Alcohol, United States Pharmacopeia (USP) Reference Standard |
| Ethanol standards 10% (v/v), 10 % (v/v) in H2O, analytical standard |
| Ethanol, absolute, for gradient elution, sales not in Germany, >=99.9% |
| Ethanol, absolute, for HPLC, sales not in Germany1, >=99.8% (GC) |
| Ethanol, absolute, semiconductor grade PURANAL(TM) (Honeywell 17826) |
| Ethyl Alcohol Absolute (Dehydrated, Synthetic) USP, BP/EP, JP Grade |
| Ethyl alcohol, Pure, 140 proof, Excise Tax-free, Permit for use required |
| Ethyl alcohol, Pure, 160 proof, Excise Tax-free, Permit for use required |
| Ethyl alcohol, Pure, 190 proof, ACS spectrophotometric grade, 95.0% |
| Specially Denatured Alcohol, 190 proof, SDA 2B-4, contains Heptanes |
| Specially Denatured Alcohol, 190 proof, SDA 2B-4, contains n-Heptane |
| Specially Denatured Alcohol, 190 proof, SDA 2B-5, contains n-Hexane |
| Specially Denatured Alcohol, 190 proof, SDA 35A, contains Ethyl acetate |
| Specially Denatured Alcohol, 190 proof, SDA 3C, contains Isopropanol |
| Specially Denatured Alcohol, 200 proof, SDA 2B-4, contains Heptanes |
| Specially Denatured Alcohol, 200 proof, SDA 2B-4, contains n-Heptane |
| Specially Denatured Alcohol, 200 proof, SDA 2B-5, contains n-Hexane |
| Specially Denatured Alcohol, 200 proof, SDA 35A, contains Ethyl acetate |
| Specially Denatured Alcohol, 200 proof, SDA 3C, contains Isopropanol |
| Alcohol Determination-Alcohol, United States Pharmacopeia (USP) Reference Standard |
| Dehydrated Alcohol, Pharmaceutical Secondary Standard; Certified Reference Material |
| Ethanol Calibration Kit, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol, absolute, reag. ISO, reag. Ph. Eur., >=99.8% (GC), liquid (clear, colorless) |
| Ethanol, puriss. p.a., ACS reagent, absolute alcohol, without additive, A15 o1, >=99.8% |
| Ethanol, puriss., meets analytical specification of Ph.??Eur., BP, 96% (v/v) |
| Ethanol, puriss., meets analytical specification of Ph.??Eur., BP, 96.0-97.2% |
| Ethanol-10, 10 mg/dL in H2O, pack of 10 x 1.2 mL ampules, certified reference material |
| Ethanol-150, 150 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-25, 25 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-300, 300 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-40, 40 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-50, 50 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-500, 500 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-80, 80 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-80, 80 mg/dL in H2O, ampule of 5 x 5 mL, certified reference material |
| Specially Denatured Alcohol, 190 proof, SDA 39C, contains Diethyl phthalate |
| Specially Denatured Alcohol, 200 proof, SDA 39C, contains Diethyl phthalate |
| 71329-38-9 |
| Ethanol, absolute, denaturated with 0.5-1.5 Vol.% 2-butanone and approx. 0.001% Bitrex (GC), >=98% (GC) |
| Ethanol, absolute, semiconductor grade PURANAL(TM) (Honeywell 17833), sales not in Germany, >=99.8% (vol.) |
| Ethanol, BioUltra, for molecular biology, >=99.8%, (absolute alcohol, without additive, A15 o1) |
| Ethanol, puriss., over molecular sieve (H2O <=0.01%), absolute alcohol, without additive, A15 o1, >=99.8% |
| Ethanol, purum, absolute ethanol, denaturated with 1% cyclohexane, A15 CYCLO1, >=99.8% (based on denaturant-free substance) |
| Ethanol, purum, absolute ethanol, denaturated with 2% 2-butanone, A15 MEK1, >=99.8% (based on denaturant-free substance) |
| Ethanol, purum, absolute ethanol, denaturated with 4.8% isopropanol, A15 IPA1, >=99.8% (based on denaturant-free substance) |
| Ethanol-100 (10 ampules/kit), 100 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-100 (5 ampules/kit), 100 mg/dL in H2O, ampule of 5 x 5 mL, certified reference material |
| Ethanol-20 (10 ampules/kit), 20 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-20 (5 ampules/kit), 20 mg/dL in H2O, ampule of 5 x 5 mL, certified reference material |
| Ethanol-200 (10 ampules/kit), 200 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-200 (5 ampules/kit), 200 mg/dL in H2O, ampule of 5 x 5 mL, certified reference material |
| Ethanol-400 (10 ampules/kit), 400 mg/dL in H2O, ampule of 10 x 1.2 mL, certified reference material |
| Ethanol-400 (5 ampules/kit), 400 mg/dL in H2O, ampule of 5 x 5 mL, certified reference material |
| Ethyl alcohol, Pure, 140 proof, meets water USP testing specifications, Excise Tax-free, Permit for use required |
| Ethyl alcohol, Pure, 190 proof, ACS reagent, meets USP testing specifications, Excise Tax-free, Permit for use required |
| Ethyl alcohol, Pure, 200 proof, ACS reagent, meets USP testing specifications, Excise Tax-free, Permit for use required |
| Specially Denatured Alcohol, 190 proof, SDA 40 (40-2), contains 0.14 % (v/v) tert-Butyl alcohol and Brucine sulfate |
| Specially Denatured Alcohol, 190 proof, SDA 40B, contains tert-Butyl alcohol and denatonium benzoate |
| Specially Denatured Alcohol, 200 proof, SDA 40 (40-2), contains 0.14 % (v/v) tert-Butyl alcohol and Brucine sulfate |
| Specially Denatured Alcohol, 200 proof, SDA 40B, contains tert-Butyl alcohol and denatonium benzoate |
|
There are more than 10 synonyms. If you wish to see them all click here.
|