| Erythrosin B |
| ERYTHROSINE |
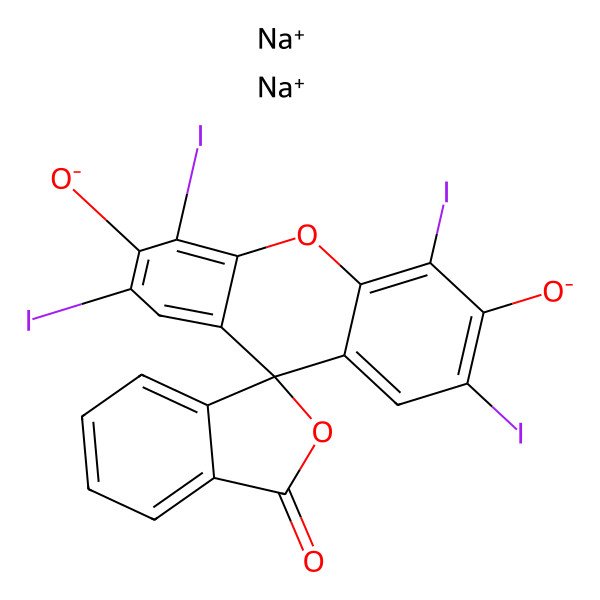
| Erythrosine B |
| C.I. Acid Red 51 |
| Erythrosine sodium (close form) |
| Erythrosine I |
| Erythrosin BS |
| Erythrosine BS |
| Erythrosine Lake |
| Erythrosine TB |
| Erythrosine 3B |
| Maple erythrosine |
| Sodium erythrosin |
| Erythrosine Extra |
| Cilefa Pink B |
| Erythrosine Bluish |
| Dolkwal erythrosine |
| Aizen Food Red 3 |
| Food Color Red 3 |
| Food Dye Red 3 |
| Food Red 3 |
| Sodium 2',4',5',7'-tetraiodo-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-3',6'-bis(olate) |
| Erythrosine TB Extra |
| FDC Red 3 dye |
| Food Red 14 |
| Calcocid erythrosine N |
| Food Red No. 3 |
| Schultz No. 887 |
| Usacert Red No. 3 |
| Aizen erythrosine |
| Hexacert Red No. 3 |
| Hexacol erythrosine BS |
| Erythrosin extra bluish |
| FD and C Red 3 |
| New Pink Bluish Geigy |
| Red Dye No. 3 |
| Canacert erythrosine BS |
| Erythrosine (indicator) |
| Caswell No. 425AB |
| FDC Red 3 |
| Erythrosine Extra Bluish |
| LB-Rot 1 |
| Erythrosine Extra Pure A |
| C.I. Food Red 14 |
| D&C Red No. 3 |
| FD and C Red No. 3 |
| Edicol Supra Erythrosine A |
| FD & C red no. 3 |
| Edicol Supra Erythrosin AS |
| Cerven kysela 51 [Czech] |
| Cerven kysela 51 |
| CCRIS 892 |
| Dye FD and C Red No. 3 |
| 1427 Red |
| 1671 Red |
| Tetraiodofluorescein sodium salt |
| Erythrosin B disodium |
| Erythrosine B (biological stain) |
| Cerven potravinarska 14 [Czech] |
| Erythrosine Extra Conc. A Export |
| Cerven potravinarska 14 |
| EINECS 240-474-8 |
| Erythrosine B-FO (biological stain) |
| Erythrosine K-FO (biological stain) |
| FD&C Red 3 |
| Erythrosine bluish (biological stain) |
| UNII-8TL7LH93FM |
| EPA Pesticide Chemical Code 120901 |
| Erythrosine sodium (close form) [USAN] |
| AI3-09094 |
| 2,4,5,7-Tetraiodofluorescein disodium salt |
| Disodium 2',4',5',7'-tetraiodofluorescein |
| DTXSID7021233 |
| E 127 |
| HSDB 7974 |
| 2',4',5',7'-Tetraiodofluoroescein disodium salt |
| 2',4',5',7'-Tetraiodofluorescein, disodium salt |
| Fluorescein, 2',4',5',7'-tetraiodo-, disodium salt |
| Spiro(isobenzofuran-1(3H),9'-(9H)xanthen)-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt |
| Disodium 2-(2,4,5,7-tetraiodo-6-oxido-3-oxoxanthen-9-yl)benzoate |
| Erythrosine B Disodium Salt |
| 9-(o-Carboxyphenyl)-6-hydroxy-2,4,5,7-tetraiodo-3H-xanthene-3-one disodium salt monohydrate |
| Disodium 9-(O-carboxyphenyl)-6-hydroxy-2,4,5,7-tetraiodo-3H-xanthen-3-one monohydrate |
| disodium;2',4',5',7'-tetraiodo-3-oxospiro[2-benzofuran-1,9'-xanthene]-3',6'-diolate |
| 3',6-Dihydroxy-2',4',5',7'-tetraiodospiro(isobenzofuran-1(3H),9'(9H)xanthen)-one disodium salt |
| Disodium 3',6'-dihydroxy-2',4',5',7'-tetraiodospiro(isobenzofuran-1(3H),9'-(9H)xanthen)-3-one |
| CI 45430 |
| Erythrosin B disodium 100 microg/mL in Acetonitrile/Water |
| DTXCID301030411 |
| NSC 36685 |
| ERYTHROSINE SODIUM (OPEN FORM) |
| Benzoic acid, 2-(6-hydroxy-2,4,5,7-tetraiodo-3-oxo-3H-xanthen-j9-yl)-, disodium salt |
| Erythrosine sodium (open form) [USAN] |
| 3',6'-Dihydroxy-2',4',5',7'-tetraiodospiro(isobenzofuran-1(3H),9'- -(9H)xanthen)-3-one, disodium salt |
| Sodium 2',4',5',7'-tetraiodo-3-oxo-3H-spiro-[isobenzofuran-1,9'-xanthene]-3',6'-bis(olate) |
| CAS-16423-68-0 |
| MFCD00144257 |
| Eritrosina |
| Benzoic acid, 2-(6-hydroxy-2,4,5,7-tetraiodo-3-oxo-3H-xanthen-9-yl)-, disodium salt |
| Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt |
| FD& Red No. 3 |
| D& Red No. 3 |
| SCHEMBL19080 |
| C20H6I4O5.2Na |
| CHEMBL2106219 |
| Erythrosin B, analytical standard |
| Tox21_202932 |
| Tox21_302085 |
| BDBM50523756 |
| AKOS015904033 |
| AKOS016010498 |
| LS-2484 |
| NSC 759227 |
| NCGC00255371-01 |
| NCGC00260478-01 |
| Erythrosin extra bluish, 87.0-100.0% |
| FT-0625707 |
| A16967 |
| 2-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl)benzoate |
| disodium 2-(2,4,5,7-tetraiodo-3,6-dioxidoxanthenium-9-yl)benzoate |
| Erythrosin extra bluish, certified by the Biological Stain Commission |
| Disodium 2- (2, 4, 5, 7- tetraiodo- 6- oxido- 3- oxoxanthen- 9- yl)benzoate |
| Erythrosin B, certified by the Biological Stain Commission, Dye content 90 % |
| Erythrosin extra bluish, for microscopy (Bact., Hist.), adsorption and fluorescent indicator |
| Fluorescein, 2',4',5',7'-tetraiodo-, disodium salt (Benzoic acid tautomeric form) |
| Disodium 2- (2, 4, 5, 7- tetraiodo- 6- oxido- 3- oxoxanthen- 9- yl)benzoate (CI 45430) |
| Espiro[isobenzofuran-1(3h),9'-[9h]xanten]-3-ona, 3',6'-dihidroxi-2',4',5',7'-tetraiodo-, sal de sodio (1:2) |
| Sodium 2',4',5',7'-tetraiodo-3-oxo-3H-spiro[isobenzo[b]furan-1,9'-xanthene]-3',6'-bis(olate) |
| Spiro(isobenzofuran-1(3H),9'-(9H)xanthen)-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, sodium salt (1:2) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|