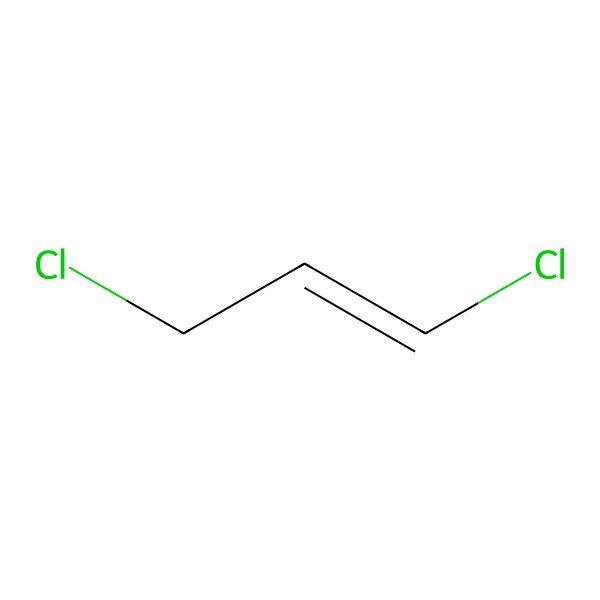
| (E)-1,3-Dichloropropene |
| 10061-02-6 |
| trans-1,3-Dichloro-1-propene |
| trans-1,3-Dichloropropene |
| Telone II |
| 1,3-Dichloropropylene |
| Telone |
| (E)-1,3-dichloroprop-1-ene |
| trans-1,3-Dichloropropylene |
| 1-Propene, 1,3-dichloro- |
| 3-Chloroallyl chloride |
| Nemex |
| Dorlone II |
| Telone C |
| Di-Trapex CP |
| Telone II-B |
| Dorlone |
| 3-Chloropropenyl chloride |
| trans-Telone |
| 1-Propene, 1,3-dichloro-, (1E)- |
| 1,3-Dichloro-2-propene |
| Vorlex 201 |
| (1E)-1,3-dichloroprop-1-ene |
| trans-3-Chloroallyl chloride |
| 1,3-Dichloropropene-1 |
| Propene, 1,3-dichloro- |
| Telone C17 |
| Telone II soil fumigant |
| D-D 92 |
| 1,3-Dichloroprop-1-ene |
| 1,3-Dichloro-1-propylene |
| alpha,gamma-Dichloropropylene |
| 1,3-Dichloropropene, trans- |
| 1-Propene, 1,3-dichloro-, (E)- |
| Caswell No. 324A |
| Dichloro-1,3 propene |
| E-1,3-Dichloropropene |
| (1E)-1,3-dichloro-1-propene |
| gamma-Chloroallyl chloride |
| 1,3-Dichloropropene [BSI:ISO] |
| DD 95 |
| NCI-C03985 |
| Tri-Form |
| 1,3-Dichloropropene, cis + trans |
| chloroallylchloride |
| Propene, 1,3-dichloro-, (E)- |
| RCRA waste number U084 |
| NSC 6202 |
| Telone IIR |
| 1,3-D |
| Dichloro-1,3 propene [ISO-French] |
| 3-dichloropropylene |
| CCRIS 955 |
| Chloroallyl Chloride |
| 1,3-Dichloropropene (technical grade) |
| Chloropropenyl chloride |
| 1, 3-Dichloropropene |
| HSDB 1109 |
| HSDB 1504 |
| 1,3-D, Dorlone |
| Telone 2000 |
| alpha-chloroallylchloride |
| chloroorpropenyl chloride |
| gamma-chloroallylchloride |
| alpha-Chloroallyl chloride |
| EINECS 208-826-5 |
| Propylene, 1,3-dichloro-, (trans) |
| RCRA waste no. U084 |
| UNII-21UG87ODPI |
| EPA Pesticide Chemical Code 029001 |
| BRN 1719556 |
| BRN 1719558 |
| 21UG87ODPI |
| CHEBI:18624 |
| D-D92 |
| UNII-9H780918D0 |
| Dichloropropene, 1,3- (Telone II) |
| 1,3-DICHLOROPROPENE (TELONE II) |
| Telone II, technical grade (without epichlorohydrin) |
| Telone II, technical grade (with 1% epichlorohydrin) |
| MFCD00000985 |
| Telone EC |
| sJPDADFHRUf`d` |
| .gamma.-Chloroallyl chloride |
| DTXSID1022057 |
| .alpha.,.gamma.-Dichloropropylene |
| Dedisol |
| Sepisol |
| Anema |
| NA2047 |
| UN2047 |
| Dedisol C |
| Dorlane II |
| 3-cloro cloroalil |
| Telone (van) |
| 1,3-Diklorpropen |
| 1,3-Dichlopropene |
| 1,3-Dichlorpropene |
| 1,3-Dicloropropeno |
| Telone 92 |
| 1 3-Dichloropropene |
| Chloroallyl C hloride |
| 3-cloro cloropropenil |
| a,?-Dichloropropylene |
| 1,3-Dicloropropileno |
| 5427-56-5 |
| Telone II, Technical |
| 1 ,3-dichloropropene |
| 1 3-Dichloropropylene |
| DPU (CHRIS Code) |
| Propeno,1,3-dicloro- |
| 13-Dichloro-1-propene |
| Dichloropropene, 1,3- |
| 1,3-dicloro-1-propeno |
| 1,3-dicloro-2-propeno |
| 1,3-Dichloropropene,c&t |
| 1 3-Dichloro-2-propene |
| trans 1,3-dichloropropene |
| (1E)-1,3-dichloropropene |
| (alpha)-chloroallyl chloride |
| (gamma)-chloroallyl chloride |
| 1-propeno, 1,3-dicloro- |
| alpha gamma-Dichloropropylene |
| Propene13-dichloro-(8CI) |
| SCHEMBL33876 |
| 1,3-Dichloropropene, E,Z- |
| BIDD:ER0524 |
| Dichloropropene, trans-1,3- |
| 1,3-Dichloro-1-propene,c&t |
| (alpha,gamma)-dichloropropylene |
| 3-D, DCP |
| CHEMBL155926 |
| DTXCID501307 |
| DTXSID2042480 |
| trans-1,3-dichloro-prop-1-ene |
| Dichloropropene, 1,3-Telone II |
| (beta)-epidichlorohydrin cis-trans |
| D-D95 |
| TELONE II, TECHNICAL GRADE |
| 1 3-dicloropropeno (grado tecnico) |
| Tox21_200235 |
| c0611 |
| LS-209 |
| LS-323 |
| 1,3-Dichloropropene (cis and trans) |
| AKOS000120933 |
| 1,3-Dichloropropene (technical-grade) |
| 1,3-DICHLOROPROPENE, (1E)- |
| 1,3-Dichloro propylene(trans-: cis-) |
| NCGC00091495-01 |
| NCGC00091495-02 |
| NCGC00257789-01 |
| 13-D |
| AS-13194 |
| CAS-542-75-6 |
| LS-123466 |
| 1,3-Dichloro-1-propene (ACD/Name 4.0) |
| 1,3-DICHLOROPROPENE (E)-FORM [MI] |
| 1,3-Dichloropropene, 90%, technical grade |
| 9H780918D0 |
| D2346 |
| S0639 |
| EN300-20393 |
| C06609 |
| C18627 |
| EC 431-460-4 |
| trans-1,3-Dichloropropene, analytical standard |
| Q161507 |
| J-000180 |
| J-524982 |
| 1,3-Dichloropropene, cis + trans, analytical standard |
| 1,3-Dichloropropene, PESTANAL(R), analytical standard |
| Telone II, technical grade (with 1% epichloro_hydrin) |
| InChI=1/C3H4Cl2/c4-2-1-3-5/h1-2H,3H2/b2-1 |
| trans-1,3-Dichloropropene, vial of 1 g, analytical standard |
|
There are more than 10 synonyms. If you wish to see them all click here.
|