| 131-11-3 |
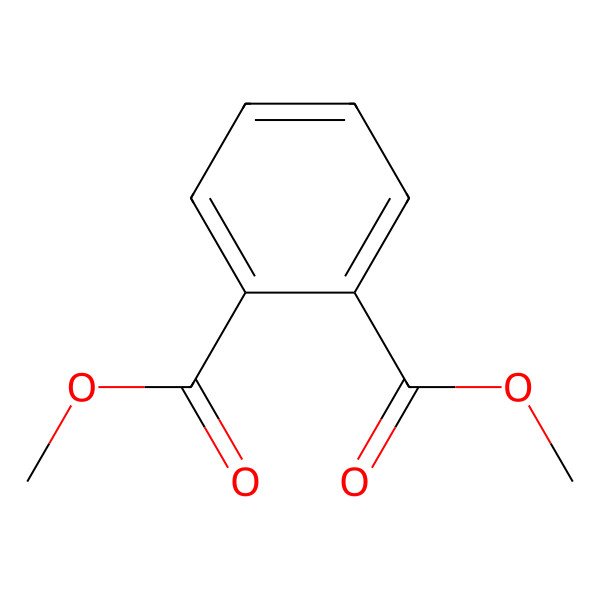
| DIMETHYLPHTHALATE |
| Avolin |
| Solvanom |
| Fermine |
| Mipax |
| Palatinol M |
| Solvarone |
| Dimethyl o-phthalate |
| Unimoll DM |
| Phthalic acid dimethyl ester |
| Repeftal |
| Methyl phthalate |
| Dimethyl 1,2-benzenedicarboxylate |
| 1,2-Benzenedicarboxylic acid, dimethyl ester |
| dimethyl benzene-1,2-dicarboxylate |
| ENT 262 |
| Phthalsaeuredimethylester |
| Phthalic acid, dimethyl ester |
| Dimethyl benzene-o-dicarboxylate |
| dimethylphtalate |
| Caswell No. 380 |
| DMF (insect repellant) |
| Dimethyl benzeneorthodicarboxylate |
| Phtalate de dimethyle |
| NSC 15398 |
| CCRIS 2674 |
| HSDB 1641 |
| UNII-08X7F5UDJM |
| RCRA waste number U102 |
| EINECS 205-011-6 |
| 08X7F5UDJM |
| NSC-15398 |
| EPA Pesticide Chemical Code 028002 |
| Dimethyl phthalate [BSI:ISO] |
| 1,2-Benzenedicarboxylic acid, 1,2-dimethyl ester |
| DTXSID3022455 |
| DMF, insect repellent |
| AI3-00262 |
| Phthalsaeuredimethylester [German] |
| DimethylPhthalate-13C2 |
| Phtalate de dimethyle [ISO-French] |
| Phthalic acid methyl ester |
| NTM |
| RCRA waste no. U102 |
| Dimethylester kyseliny ftalove [Czech] |
| Benzenedicarboxylic acid, dimethyl ester |
| Dimethylester kyseliny ftalove |
| CHEBI:4609 |
| CHEMBL323348 |
| DTXCID502455 |
| Phthalic acid, bis-methyl ester |
| Dimethyl 1,2-benzendicarboxylate |
| EC 205-011-6 |
| NCGC00090692-02 |
| Dimethyl phthalate 5000 microg/mL in Methanol |
| 1,2-benzenedicarboxylic acid 1,2-dimethyl ester |
| Dimethyl phthalate, >=99% |
| DIMETHYL PHTHALATE (II) |
| DIMETHYL PHTHALATE [II] |
| DIMETHYL PHTHALATE (MART.) |
| DIMETHYL PHTHALATE [MART.] |
| CAS-131-11-3 |
| PHTHALIC ACID DIMETHYL ESTER (D6) |
| Dimethyl phthalate, PESTANAL(R), analytical standard |
| Dimetylftalat |
| Density Standard 1191 kg/m3 |
| Bisoflex DMP |
| Kemester DMP |
| Kodaflex DMP |
| 1,dimethyl ester |
| Ftalato di metile |
| Uniplex 110 |
| Caswell No 380 |
| Dimethyl orthophthalate |
| phthalsauredimethylester |
| 1,2-dimethyl benzene-1,2-dicarboxylate |
| Dimethyl phthalate [C] |
| DTL (CHRIS Code) |
| 1,2-dimethyl phthalate |
| Dimethyl phthalate, 99% |
| Dimethyl phthalate [USP] |
| DMP (Dimethyl phthalate) |
| WLN: 1OVR BVO1 |
| SCHEMBL34630 |
| Dimethyl phthalate, AR,99% |
| Dimethyl phthalate, CP,99% |
| MLS002177801 |
| BIDD:ER0349 |
| Phtalate de dimthyle (ortho-) |
| DIMETHYL PHTHALATE [MI] |
| DIMETHYL PHTHALATE [ISO] |
| DIMETHYL PHTHALATE [HSDB] |
| DIMETHYL PHTHALATE [INCI] |
| FIFRA 028002 |
| AMY40794 |
| DIMETHYL PHTHALATE [WHO-DD] |
| HY-N7106 |
| NSC15398 |
| Tox21_113536 |
| Tox21_202145 |
| Tox21_301045 |
| 1,2-bencenodicarboxilato de dimetilo |
| BDBM50090983 |
| LS-737 |
| MFCD00008425 |
| s5378 |
| STL283931 |
| AKOS008969337 |
| CCG-266531 |
| DB13336 |
| USEPA/OPP Pesticide Code: 028002 |
| Dimethyl ester of 1,2-benzenedicarboxyli |
| NCGC00090692-01 |
| NCGC00090692-03 |
| NCGC00090692-04 |
| NCGC00090692-05 |
| NCGC00090692-06 |
| NCGC00254947-01 |
| NCGC00259694-01 |
| 1,2 Benzene dicarboxylacid, dimethylester |
| BS-20466 |
| SMR000777937 |
| 1,2-Benzendicarboxylic acid, dimethyl ester |
| 1,2-Benzenedicarboxylic acid, demethyl ester |
| benzene-1,2-dicarboxylic acid dimethyl ester |
| CS-0013572 |
| FT-0625095 |
| P0302 |
| EN300-18366 |
| Dimethyl phthalate, SAJ special grade, >=99.0% |
| Q423551 |
| J-005938 |
| Z57902306 |
| Density Standard 1191 kg/m3, H&D Fitzgerald Ltd. Quality |
| Phthalic acid, bis-methyl ester 1000 microg/mL in Acetonitrile |
| BENZENE,1,2-DICARBOXYLIC ACID,DIMETHYL ESTER (PHTHALIC ACID,DIMETHYL ESTER) |
| InChI=1/C10H10O4/c1-13-9(11)7-5-3-4-6-8(7)10(12)14-2/h3-6H,1-2H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|