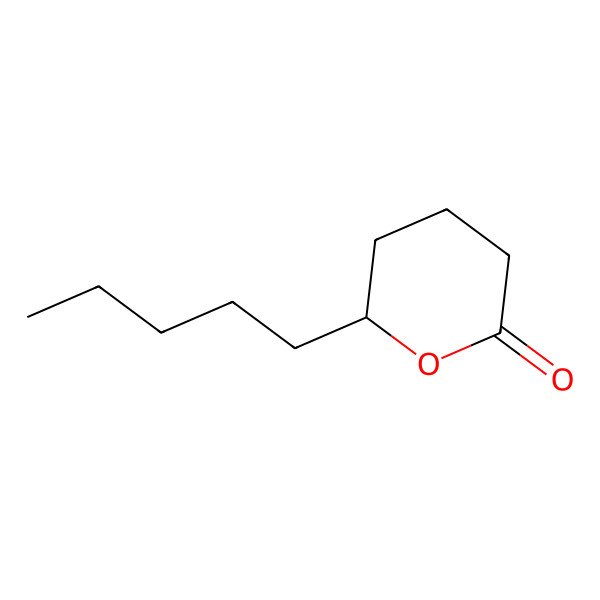
| 705-86-2 |
| 6-Pentyltetrahydro-2H-pyran-2-one |
| 5-Decanolide |
| 2H-Pyran-2-one, tetrahydro-6-pentyl- |
| 6-pentyloxan-2-one |
| Decan-5-olide |
| delta-Decanolactone |
| Decanolide-1,5 |
| 5-Decalactone |
| Amyl-delta-valerolactone |
| 5-Pentyl-5-pentanolide |
| 5-Hydroxydecanoic acid delta-lactone |
| FEMA No. 2361 |
| delta-Pentyl-delta-valerolactone |
| (+/-)-5-Decanolide |
| Tetrahydro-6-pentyl-2H-pyran-2-one |
| .delta.-Decalactone |
| 5-Amyl-5-hydroxypentanoic acid lactone |
| AI3-36028 |
| .delta.-Amylvalerolactone |
| MFCD00006649 |
| (R)-(+)-delta-Decanolactone |
| CNA0S5T234 |
| Tetrahydro-6-pentylpyran-2-one |
| DTXSID0044496 |
| CHEBI:87327 |
| (S)-(-)-DELTA-DECANOLACTONE |
| Decanoic acid, 5-hydroxy-, .delta.-lactone |
| delta-Decalactone (natural) |
| d-decalactone |
| EINECS 211-889-1 |
| BRN 0117520 |
| UNII-CNA0S5T234 |
| .delta.-Decanolide |
| delta-Caprinolactone |
| 6-pentylvalerolactone |
| .delta.-Decanolactone |
| delta-Amylvalerolactone |
| (+/-)-delta-Pentyl-delta-valerolactone |
| DECALACTONE DELTA |
| .DELTA.-LACTONE |
| delta - DECALACTONE |
| (+/-)-6-Pentyltetrahydro-2H-pyran-2-one |
| 5-Hydroxydecanoic lactone |
| .DELTA.-LACTONE- |
| Amyl-.delta.-valerolactone |
| 5-Decanolide, >=98% |
| EC 211-889-1 |
| (R)-(+)delta-Decalactone |
| laquo deltaRaquo -decanolide |
| laquo deltaRaquo -decalactone |
| 5-17-09-00090 (Beilstein Handbook Reference) |
| SCHEMBL114875 |
| 5-Hydroxydecanoic acid lactone |
| laquo deltaRaquo -decanolactone |
| DELTA-DECALACTONE [FCC] |
| CHEMBL3182189 |
| DELTA-DECALACTONE [INCI] |
| DTXCID8024496 |
| 6-pentyl-tetrahydro-pyran-2-one |
| FEMA 2361 |
| (RS)-.DELTA.-DECALACTONE |
| .DELTA.-DECALACTONE [MI] |
| laquo deltaRaquo -amylvalerolactone |
| .DELTA.-DECALACTONE [FHFI] |
| Amyl-laquo deltaRaquo -valerolactone |
| LT laquo deltaRaquo GT -decalactone |
| Tox21_301878 |
| AC2158 |
| BBL027599 |
| LMFA07040028 |
| STL373782 |
| (+/-)-.DELTA.-DECALACTONE |
| delta-Decalactone, analytical standard |
| 6-Pentyltetrahydro-2H-pyran-2-one # |
| AKOS015914990 |
| 5-Hydroxydecanoic acid .delta.-lactone |
| CS-W017695 |
| DS-3354 |
| HY-W016979 |
| delta-Decalactone, >=98%, FCC, FG |
| NCGC00255981-01 |
| CAS-705-86-2 |
| SY015319 |
| D1416 |
| FT-0624501 |
| 5-Decanolide (laquo deltaRaquo -decalactone) |
| CAPRIC ACID, .DELTA.-HYDROXY-, LACTONE |
| delta-Decalactone, natural, >=98%, FCC, FG |
| EN300-203358 |
| FEMA NO. 4611, .DELTA.-DECALACTONE- |
| 5-Hydroxydecanoic acid laquo deltaRaquo -lactone |
| W-104548 |
| Decanoic acid, 5-hydroxy-, laquo deltaRaquo -lactone |
| Q27159530 |
| delta-Decanolactone, (+/-)-delta-Pentyl-delta-valerolactone |
|
There are more than 10 synonyms. If you wish to see them all click here.
|