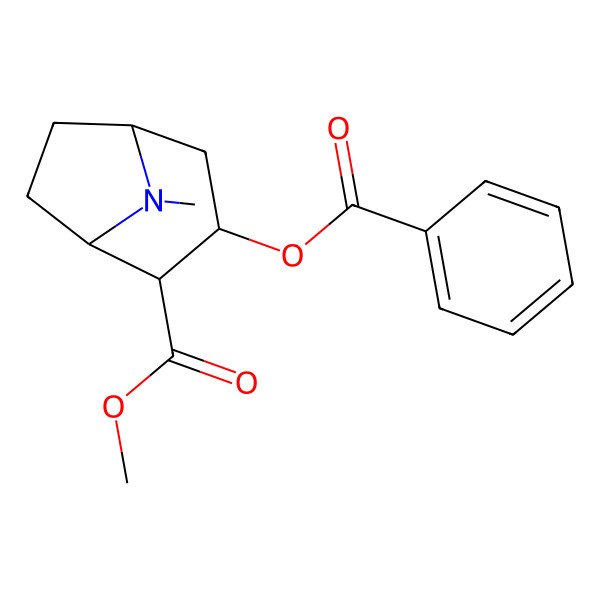
| Benzoylmethylecgonine |
| Neurocaine |
| Kokain |
| Cocain |
| L-Cocaine |
| Cocaina |
| beta-Cocain |
| (-)-Cocaine |
| cocainum |
| Methyl Benzoylecgonine |
| Coke |
| l-Cocain |
| Eritroxilina |
| Erytroxylin |
| Bernies |
| Kokayeen |
| Burese |
| Corine |
| Kokan |
| Dama blanca |
| Pimp's drug |
| 1-Cocaine |
| White girl or lady |
| Star-spangled powder |
| Leaf |
| Snow |
| Nose candy |
| Cocaine free base |
| Cocaine, l- |
| C Carrie |
| 50-36-2 |
| Blow [Street Name] |
| Girl [Street Name] |
| Lady [Street Name] |
| Rock [Street Name] |
| Toot [Street Name] |
| Cecil [Street Name] |
| Flake [Street Name] |
| (r)-(-)-cocaine |
| Bernice [Street Name] |
| CHEBI:27958 |
| HSDB 6469 |
| Ecgonine, methyl ester, benzoate (ester) |
| Gold dust [Street Name] |
| 2-beta-Carbomethoxy-3-beta-benzoxytropane |
| Happy dust [Street Name] |
| UNII-I5Y540LHVR |
| I5Y540LHVR |
| Bernice |
| Cholly |
| Cecil |
| Girl |
| 2beta-Carbomethoxy-3beta-benzoxytropane |
| Happy dust |
| Gold dust |
| IDS-NC-004 |
| Star dust |
| 3-Tropanylbenzoate-2-carboxylic acid methyl ester |
| Cholly [Street Name] |
| Cocaine [USP:BAN] |
| (1R,2R,3S,5S)-2-Methoxycarbonyltropan-3-yl benzoate |
| 2-beta-Tropanecarboxylic acid, 3-beta-hydroxy-, methyl ester, benzoate (ester) |
| EINECS 200-032-7 |
| RX0041 |
| Star dust [Street Name] |
| Green gold [Street Name] |
| RX-0041 |
| Happy trails [Street Name] |
| Methyl 3beta-hydroxy-1alphaH,5alphaH-tropane-2beta-carboxylate benzoate (ester) |
| Jam |
| DTXSID2038443 |
| Crack cocaine |
| 2-Methyl-3beta-hydroxy-1alphaH,5alphaH-tropane-2beta-carboxylate benzoate (ester) |
| 3beta-Hydroxy-1alphaH,5alphaH-tropane-2beta-carboxylic acid methyl ester benzoate |
| 1-alpha-H,5-alpha-H-Tropane-2-beta-carboxylic acid, 3-beta-hydroxy-, methyl ester, benzoate |
| 3-(Benzoyloxy)-8-methyl-8-azabicyclo-(3.2.1)octane-2-carboxylic acid methyl ether |
| methyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| Methyl 3-beta-hydroxy-1-alpha-H,5-alpha-H-tropane-2-beta-carboxylate benzoate (ester) |
| Blow (Street Name) |
| Girl (Street Name) |
| Lady (Street Name) |
| Rock (Street Name) |
| Toot (Street Name) |
| Ecgonine methyl ester benzoate (ester) |
| Cecil (Street Name) |
| COC |
| Flake (Street Name) |
| (1R,2R,3S,5S)-2-(methoxycarbonyl)tropan-3-yl benzoate |
| Cholly (Street Name) |
| Cocaine (USP:BAN) |
| Bernice (Street Name) |
| Crack |
| Flake |
| Blow |
| Lady |
| Toot |
| Happy trails |
| COCAINE (MART.) |
| COCAINE [MART.] |
| Green gold |
| Gold dust (Street Name) |
| Star dust (Street Name) |
| 3beta-Hydroxy-2beta-tropanecarboxylic acid methyl ester benzoate (ester) |
| 8-Azabicyclo(3.2.1)octane-2-carboxylic acid, 3-(benzoyloxy)-8-methyl-, methyl ester, (1R-(exo,exo))- |
| Green gold (Street Name) |
| Happy dust (Street Name) |
| Happy trails (Street Name) |
| methyl [1R-(exo,exo)]-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| C" Carrie |
| COCAINE (USP MONOGRAPH) |
| COCAINE [USP MONOGRAPH] |
| Sleighride |
| Badrock |
| Bazooka |
| Blizzard |
| Cabello |
| Charlie |
| Cocktail |
| Goofball |
| Moonrocks |
| Blast |
| Candy |
| Caviar |
| Freeze |
| Heaven |
| Snort |
| Trails |
| Coca |
| Cola |
| Hell |
| Toke |
| Yeyo |
| beta-Cocaine |
| Bouncing Powder |
| Chicken Scratch |
| Happy powder |
| Florida Snow |
| Sweet Stuff |
| Prime Time |
| Foo Foo |
| Kibbles n' Bits |
| Snow (birds) |
| G-Rock |
| [1R-(exo,exo)]-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid, methyl ester |
| METHYL (1R,2R,3S,5S)-3-(BENZOYLOXY)-8-METHYL-8-AZABICYCLO(3.2.1) OCTANE-2-CARBOXYLATE |
| methyl (1R,2R,3S,5S)-8-methyl-3-[(phenylcarbonyl)oxy]-8-azabicyclo[3.2.1]octane-2-carboxylate |
| Ecgonine methyl ester benzoate |
| methyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo(3.2.1)octane-2-carboxylate |
| methyl (1R-(exo,exo))-3-(benzoyloxy)-8-methyl-8-azabicyclo(3.2.1)octane-2-carboxylate |
| (1R-(exo,exo))-3-(benzoyloxy)-8-methyl-8-azabicyclo(3.2.1)octane-2-carboxylic acid, methyl ester |
| Cocaine (-) |
| 1i7z |
| COCAINE [VANDF] |
| COCAINUM [HPUS] |
| COCAINE [HSDB] |
| COCAINE [MI] |
| COCAINE [WHO-DD] |
| Epitope ID:158626 |
| SCHEMBL21930 |
| CHEMBL370805 |
| GTPL2286 |
| DEA No. 9041 |
| BDBM22418 |
| Cocaine, 1mg/ml in Acetonitrile |
| 1q72 |
| ZPUCINDJVBIVPJ-LJISPDSOSA-N |
| DTXCID501030562 |
| Cocaine 0.1 mg/ml in Acetonitrile |
| Cocaine 1.0 mg/ml in Acetonitrile |
| AKOS015965554 |
| DB00907 |
| C01416 |
| Q41576 |
| [1R-(exo,exo)]-3-(Benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic Acid |
| 3.BETA.-HYDROXY-2.BETA.-TROPANECARBOXYLIC ACID METHYL ESTER BENZOATE (ESTER) |
| METHYL 3B-HYDROXY-1.ALPHA.H,5.ALPHA.H-TROPANE-2.BETA.-CARBOXYLATE BENZOATE (ESTER) |
| METHYL 3B-HYDROXY-1alphaH,5alphaH-TROPANE-2beta-CARBOXYLATE BENZOATE (ESTER) |
| 1-alpha-H,5-alpha-H-Tropane-2-beta-carboxylic acid, 3-beta-hydroxy-, methyl ester, benzoate (ester) (8CI) |
| 8-Azabicyclo[3.2.1]octane-2-carboxylic acid, 3-(benzoyloxy)-8-methyl-, methyl ester, (1R,2R,3S,5S)- (9CI) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|