Cephalomannine
| Internal ID | ff0514b9-8f7b-4d50-bd8d-4aef005c39cb |
| Taxonomy | Lipids and lipid-like molecules > Prenol lipids > Diterpenoids > Taxanes and derivatives |
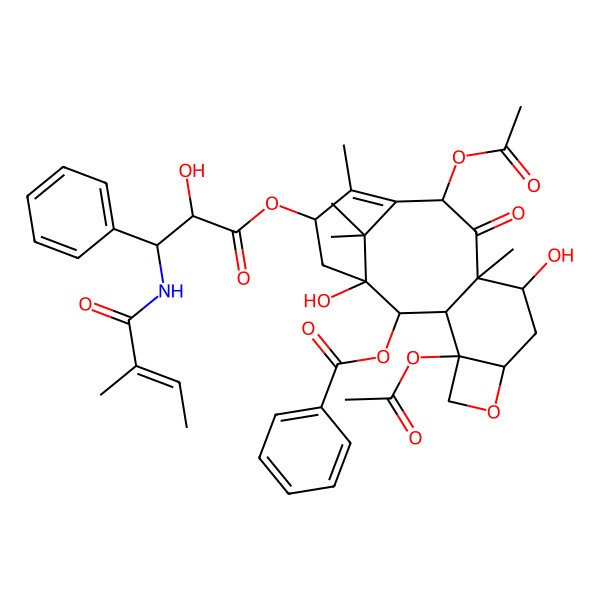
| IUPAC Name | [(1S,2S,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-1,9-dihydroxy-15-[(2R,3S)-2-hydroxy-3-[[(E)-2-methylbut-2-enoyl]amino]-3-phenylpropanoyl]oxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate |
| SMILES (Canonical) | CC=C(C)C(=O)NC(C1=CC=CC=C1)C(C(=O)OC2CC3(C(C4C(C(CC5C4(CO5)OC(=O)C)O)(C(=O)C(C(=C2C)C3(C)C)OC(=O)C)C)OC(=O)C6=CC=CC=C6)O)O |
| SMILES (Isomeric) | C/C=C(\C)/C(=O)N[C@@H](C1=CC=CC=C1)[C@H](C(=O)O[C@H]2C[C@]3([C@H](C4[C@@]([C@H](C[C@@H]5[C@]4(CO5)OC(=O)C)O)(C(=O)[C@@H](C(=C2C)C3(C)C)OC(=O)C)C)OC(=O)C6=CC=CC=C6)O)O |
| InChI | InChI=1S/C45H53NO14/c1-9-23(2)39(52)46-33(27-16-12-10-13-17-27)34(50)41(54)58-29-21-45(55)38(59-40(53)28-18-14-11-15-19-28)36-43(8,30(49)20-31-44(36,22-56-31)60-26(5)48)37(51)35(57-25(4)47)32(24(29)3)42(45,6)7/h9-19,29-31,33-36,38,49-50,55H,20-22H2,1-8H3,(H,46,52)/b23-9+/t29-,30-,31+,33-,34+,35+,36?,38-,43+,44-,45+/m0/s1 |
| InChI Key | DBXFAPJCZABTDR-UJLUYDJNSA-N |
| Popularity | 157 references in papers |
| Molecular Formula | C45H53NO14 |
| Molecular Weight | 831.90 g/mol |
| Exact Mass | 831.34660536 g/mol |
| Topological Polar Surface Area (TPSA) | 221.00 Ų |
| XlogP | 2.00 |
| CHEBI:3536 |
| 71610-00-9 |
| Taxol B |
| MLS001097651 |
| CHEMBL1397662 |
| SCHEMBL12887366 |
| Cephalomannine, analytical standard |
| HMS2267G09 |
| AKOS015960518 |
| AKOS037515801 |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL1293277 | O15118 | Niemann-Pick C1 protein |
707.9 nM |
Potency |
via Super-PRED
|
| CHEMBL1293294 | P51151 | Ras-related protein Rab-9A |
100 nM |
Potency |
via Super-PRED
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL221 | P23219 | Cyclooxygenase-1 | 99.87% | 90.17% |
| CHEMBL2581 | P07339 | Cathepsin D | 98.61% | 98.95% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 97.60% | 91.11% |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha | 97.30% | 85.14% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 94.64% | 86.33% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 94.08% | 95.56% |
| CHEMBL3091268 | Q92753 | Nuclear receptor ROR-beta | 93.07% | 95.50% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 92.30% | 96.09% |
| CHEMBL3038477 | P67870 | Casein kinase II alpha/beta | 90.76% | 99.23% |
| CHEMBL340 | P08684 | Cytochrome P450 3A4 | 90.47% | 91.19% |
| CHEMBL2535 | P11166 | Glucose transporter | 90.21% | 98.75% |
| CHEMBL5028 | O14672 | ADAM10 | 90.20% | 97.50% |
| CHEMBL1951 | P21397 | Monoamine oxidase A | 90.04% | 91.49% |
| CHEMBL3475 | P05121 | Plasminogen activator inhibitor-1 | 90.04% | 83.00% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 89.66% | 94.45% |
| CHEMBL1806 | P11388 | DNA topoisomerase II alpha | 89.50% | 89.00% |
| CHEMBL2035 | P08912 | Muscarinic acetylcholine receptor M5 | 89.49% | 94.62% |
| CHEMBL3130 | O00329 | PI3-kinase p110-delta subunit | 87.77% | 96.47% |
| CHEMBL4302 | P08183 | P-glycoprotein 1 | 87.66% | 92.98% |
| CHEMBL1075094 | Q16236 | Nuclear factor erythroid 2-related factor 2 | 87.23% | 96.00% |
| CHEMBL3145 | P42338 | PI3-kinase p110-beta subunit | 87.12% | 98.75% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 86.18% | 97.09% |
| CHEMBL1821 | P08173 | Muscarinic acetylcholine receptor M4 | 85.93% | 94.08% |
| CHEMBL4267 | P37173 | TGF-beta receptor type II | 85.17% | 88.18% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 85.00% | 99.17% |
| CHEMBL2716 | Q8WUI4 | Histone deacetylase 7 | 84.66% | 89.44% |
| CHEMBL4026 | P40763 | Signal transducer and activator of transcription 3 | 84.49% | 82.69% |
| CHEMBL1293267 | Q9HC97 | G-protein coupled receptor 35 | 81.69% | 89.34% |
| CHEMBL4478 | Q00975 | Voltage-gated N-type calcium channel alpha-1B subunit | 81.17% | 97.14% |
| CHEMBL4208 | P20618 | Proteasome component C5 | 81.12% | 90.00% |
| CHEMBL2095172 | P14867 | GABA-A receptor; alpha-1/beta-2/gamma-2 | 80.86% | 92.67% |
| CHEMBL2964 | P36507 | Dual specificity mitogen-activated protein kinase kinase 2 | 80.72% | 80.00% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| Corylus avellana |
| Dioscorea communis |
| Taxus baccata |
| Taxus brevifolia |
| Taxus canadensis |
| Taxus cuspidata |
| Taxus wallichiana |
| Taxus wallichiana var. chinensis |
| PubChem | 5281819 |
| LOTUS | LTS0164359 |
| wikiData | Q27106124 |