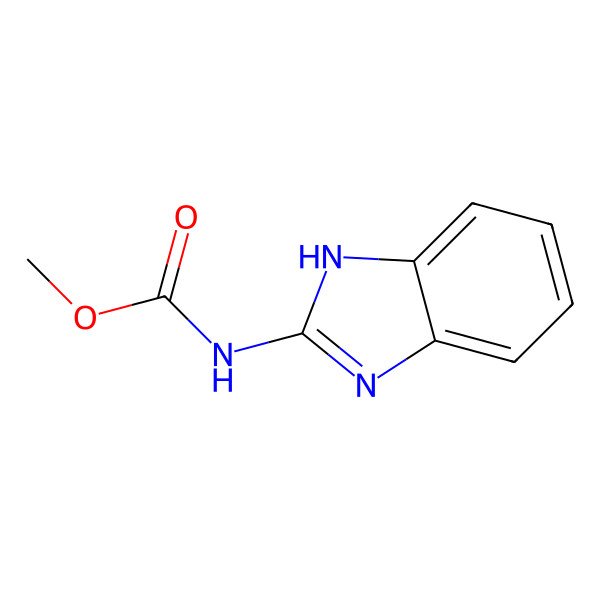
| 10605-21-7 |
| Carbendazole |
| Bavistin |
| Mecarzole |
| Thicoper |
| Carbendazime |
| Bavistan |
| Carbendazol |
| Derosal |
| Funaben |
| Medamine |
| Carbendazym |
| Equitdazin |
| Garbenda |
| Kemdazin |
| Supercarb |
| Agrizim |
| Battal |
| Bengard |
| Bitosen |
| Custos |
| Delsene |
| Karben |
| Kolfugo |
| Stempor |
| BMK (fungicide) |
| Methyl 2-benzimidazolecarbamate |
| Myco |
| Methyl benzimidazol-2-ylcarbamate |
| Bavistin 3460 |
| carbendazine |
| Falicarben |
| Fungisol |
| Pillarstin |
| Triticol |
| Stein |
| Spin |
| Bercema-Bitosen |
| Kolfugo Extra |
| methyl N-(1H-benzimidazol-2-yl)carbamate |
| Preventol BCM |
| Antibac MF |
| Carben VL |
| Funaben 3 |
| BCM (fungicide) |
| Carbamic acid, 1H-benzimidazol-2-yl-, methyl ester |
| Jkatein |
| methyl 1H-benzo[d]imidazol-2-ylcarbamate |
| Funaben 50 |
| Benzimidazolecarbamic |
| IPO Y |
| 2-MBC |
| Methyl benzimidazolylcarbamate |
| Preparation G 665 |
| METHYL 1H-BENZIMIDAZOL-2-YLCARBAMATE |
| A 118 (pesticide) |
| carbendazin |
| carbendazyme |
| mekarzole |
| 2-(Methoxycarbamoyl)benzimidazole |
| Mercarzole |
| 2-(Carbomethoxyamino)benzimidazole |
| Kolfugo 25 FW |
| Methyl 1H-benzimidazole-2-carbamate |
| 1H-Benzimidazole-2-carbamic acid, methyl ester |
| 2-(Methoxycarbonylamino)-benzimidazole |
| BAS 67054F |
| Lignasan |
| 2-(Methoxycarbonylamino)benzimidazole |
| 2-Benzimidazolecarbamic acid, methyl ester |
| Bavistine |
| Derroprene |
| Jkstein |
| Zhiweiling |
| Methyl N-2-benzimidazolecarbamate |
| 1H-Benzimidazol-2-ylcarbamic acid methyl ester |
| BAS-3460 |
| CTR 6669 |
| Bavistin FL |
| EK 578 |
| HOE 17411 |
| CCRIS 1553 |
| Spin (pesticide) |
| Benzimidazole-2-carbamic acid, methyl ester |
| Carbendazim [BSI:ISO] |
| 2-(Methoxy-carbonylamino)-benzimidazol |
| G 665 |
| Olgin (fungicide) |
| Bavistin 25SD |
| Bavistin 50SD |
| HSDB 6581 |
| BMK (VAN) |
| Delsene 10 |
| Derosal 60PM |
| Kolfugo 25FW |
| Methyl benzimidazolecarbamate |
| IPO-1250 |
| U 32104 |
| BA 67054F |
| Fungoxan |
| methyl 2-benzimidazil carbamate |
| Protek |
| Sarfun |
| Subeej |
| Methyl 2-benzimidazolylcarbamate |
| BCM |
| methyl-2-benzimidazole carbamate |
| methylbenzimidazole-2-ylcarbamate |
| BAS 3460F |
| Benzimidazole carbamate de methyle |
| benzimidazolecarbamate methyl ester |
| BAS 3460 F |
| Carbendazim D3 |
| MBC (VAN) |
| Carbendazime [ISO-French] |
| IPO 1250 |
| methoxybenzimidazole-2-carbamic acid |
| Karben flo Stefes |
| BMK |
| EINECS 234-232-0 |
| Karben Stefes Flo |
| methyl-N-(2-benzimidazolyl)carbamate |
| BAS-67054 |
| FB-642 |
| Carbendazim (MBC) |
| Carbendazim [ISO] |
| EPA Pesticide Chemical Code 115001 |
| EPA Pesticide Chemical Code 128872 |
| NSC 109874 |
| NSC-109874 |
| UNII-H75J14AA89 |
| 1H-Benzimidazol-2-ylcarbamic acid, methyl ester |
| 2-[(Methoxycarbonyl)amino]benzimidazole |
| 2-Methyl benzimidazolecarbamate |
| C9H9N3O2 |
| CHEBI:3392 |
| 2-benzimidazolecarbamic acid methyl ester |
| 105268-95-9 |
| DTXSID4024729 |
| U-32.104 |
| Methyl 1H-benzimidazolylcarbamate |
| BAS-67054F |
| FB642 |
| H75J14AA89 |
| 2-(Methoxycarboxamido)benzimidazole |
| A 118 |
| BAS 3460 |
| CTR-6669 |
| EK-578 |
| RCRA waste no. U372 |
| Carbamic acid, N-1H-benzimidazol-2-yl-, methyl ester |
| CHEMBL70971 |
| Benzimidazole carbamate de methyle [French] |
| MLS002701961 |
| DTXCID004729 |
| Methyl 1H-benzimidazol-2-ylcarbamate (9CI) |
| 2-(Methoxy-carbonylamino)-benzimidazol [German] |
| Carbamic acid, 1H-benzimidazolyl-, methyl ester |
| 1H-Benzimidazol-2-yl-carbamic acid, methyl ester |
| HOE-17411 |
| NSC109874 |
| 2-Bezimidazolecarbamic acid methyl ester |
| A118 |
| methyl N-(1H-1,3-benzodiazol-2-yl)carbamate |
| METHYL N-1H-BENZIMIDAZOL-2-YLCARBAMATE |
| 37953-07-4 |
| 39413-19-9 |
| KID PEST PROJECT (CARBENDAZIM) (SEE ALSO CARBENDAZIM) |
| Carbamic acid, N-1H-benzimidazol-2-yl-, methyl ester;Methyl 1H-benzo[d]imidazol-2-ylcarbamate |
| CAS-10605-21-7 |
| ALBENDAZOLE IMPURITY E (EP IMPURITY) |
| ALBENDAZOLE IMPURITY E [EP IMPURITY] |
| 2-((METHOXYCARBONYL)AMINO)BENZIMIDAZOLE |
| 2-(Methoxy-carbonylamino)-benzimidazol (GERMAN) |
| carb |
| Carbenzadime |
| Carbate |
| Cekudazim |
| Benomyl metabolite |
| Carbendazol, JMAF |
| MBC (Carbendazim) |
| Tripart defensor FL |
| N-benzimidazol-2-ylmethoxycarboxamide |
| Carbendazim, 97% |
| MCAB |
| Turfclear (Salt/Mix) |
| BMC? |
| CARBENDAZIM [MI] |
| ChemDivAM_000201 |
| G-665 |
| ChemDiv1_000052 |
| 1h-benzimidazole-2-carbamic acid methyl ester |
| CARBENDAZIM [HSDB] |
| Methyl benzimidazolycarbamate |
| SCHEMBL21051 |
| CARBENDAZIM [WHO-DD] |
| N-1H-Benzimidazol-2-yl-carbamic Acid Methyl Ester |
| BIDD:ER0282 |
| methyl-2-benzimidazolecarbamate |
| methoxycarbonylaminobenzimidazole |
| Carbendazim, analytical standard |
| N-2-(benzimidazolyl) carbamate |
| 2-carbomethoxyamino-benzimidazole |
| methyl benzimidazole-2-carbamate |
| SCHEMBL19926051 |
| HMS587C08 |
| methyl 2-benzimidazolyl-carbamate |
| methyl benzimidazole-2-ylcarbamate |
| methyl benzimidazol-2-yl carbamate |
| AMY22465 |
| Methyl bendimidazole-2-yl-carbamate |
| methyl benzimidazole-2-yl carbamate |
| Methyl-alpha-benzimidazole Carbamate |
| Tox21_202295 |
| Tox21_300478 |
| 2-(carbomethoxy-amino)-benzimidazole |
| BAS 67054 |
| BDBM50116313 |
| FB 642 |
| LS-418 |
| Methyl 1H-2-benzimidazolecarbaminate |
| Methyl 1H-benzemedazol-2-ylcarbamate |
| Methyl N-benzimidazol-2-yl-carbamate |
| MFCD00055390 |
| Carbendazim 50 microg/mL in Methanol |
| N-2-(BENZIMIDAZOLYL)CARBAMATE |
| AKOS002384358 |
| methyl-1h-benzimidazol-2-yl carbamate |
| Carbendazim 100 microg/mL in Methanol |
| CCG-101273 |
| CS-8030 |
| DB13009 |
| KS-5360 |
| 2-(Methoxy-carbonylamino)-benzimidazole |
| methyl (1h-benzimidazol-2-yl)carbamate |
| USEPA/OPP Pesticide Code: 128872 |
| NCGC00090706-01 |
| NCGC00090706-02 |
| NCGC00090706-03 |
| NCGC00090706-04 |
| NCGC00254328-01 |
| NCGC00259844-01 |
| 2-Benzimidazolecarcamic Acid Methyl Ester |
| AC-10590 |
| BP-21318 |
| HY-13582 |
| NCI60_000240 |
| SMR000304463 |
| 1H-Benzimidazol-2-ylcarbamicacidmethylester |
| Methyl 1H-benzimidazol-2-ylcarbamate, 9CI |
| FT-0602933 |
| FT-0664246 |
| S3676 |
| Carbendazim, PESTANAL(R), analytical standard |
| E78626 |
| EN300-180487 |
| A801368 |
| AF-962/00515023 |
| N-1H-Benzimidazol-2-ylcarbamic acid methyl ester |
| Q418607 |
| SR-01000390861 |
| (1H-Benzoimidazol-2-yl)-carbamic acid methyl ester |
| Carbamic acid, 1H-benzimidazol-2-yl, methyl ester |
| J-001536 |
| SR-01000390861-1 |
| Carbendazim, certified reference material, TraceCERT(R) |
| F0266-0908 |
| Z752774256 |
| Fenbendazole impurity A, European Pharmacopoeia (EP) Reference Standard |
| 102040-01-7 |
| 110342-67-1 |
| 212384-28-6 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|