| 514-78-3 |
| Orobronze |
| Carophyll Red |
| Canthaxanthine |
| Cantaxanthin |
| beta,beta-Carotene-4,4'-dione |
| Food orange 8 |
| Roxanthin Red 10 |
| Cantaxanthine |
| L-Orange 7 |
| 4,4'-Dioxo-beta-carotene |
| Canthaxanthin (trans) |
| CanthaXanthin, Powder |
| CI 40850 |
| all-trans,beta-Carotene-4,4'-dione |
| CHEBI:3362 |
| DTXSID0022727 |
| E 161g;all-trans-Canthaxanthin |
| 4C3C6403MU |
| NSC-374110 |
| NCGC00095896-01 |
| C.I. Food Orange 8 |
| All-trans-Canthaxanthin |
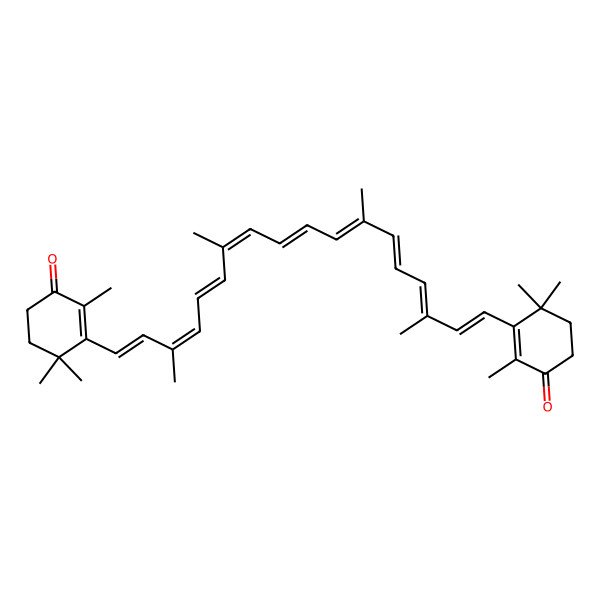
| 3,3'-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,4,4-trimethylcyclohex-2-en-1-one) |
| Carotene-4,4'-dione, beta- |
| CCRIS 3276 |
| E 161g |
| EINECS 208-187-2 |
| NSC 374110 |
| Ro 1-9915 |
| E 161 G |
| BRN 1898520 |
| beta-CAROTENE-4,4'-DIONE, all-trans- |
| UNII-4C3C6403MU |
| CANTHAXANTHIN (euglenanone) |
| MFCD00016364 |
| CANTHA |
| KANTAKISANTIN |
| Canthaxanthin, tech. |
| LUCANTIN RED |
| Isomer of Canthaxanthin |
| 4,4'-Diketo-b-carotene |
| CI-FOOD ORANGE 8 |
| CANTHAXANTHIN [MI] |
| beta-Carotin-4,4?-dione |
| CANTHAXANTHIN [FCC] |
| 4,4'-Diketo-beta-carotene |
| SCHEMBL19618 |
| 4-07-00-02680 (Beilstein Handbook Reference) |
| CANTHAXANTHIN [MART.] |
| E161g |
| INS NO.161G |
| SPECTRUM1504204 |
| CANTHAXANTHIN [WHO-DD] |
| DTXCID002727 |
| INS-161G |
| CHEMBL1329004 |
| SCHEMBL12920083 |
| CANTHAXANTHIN (E 161G) |
| FDSDTBUPSURDBL-DKLMTRRASA-N |
| CI 40850 [INCI] |
| HMS2089K15 |
| HY-B1960 |
| Tox21_111533 |
| all-trans-beta-carotene-4,4'-dione |
| Canthaxanthin, >=95.0% (HPLC) |
| E-161G |
| LMPR01070264 |
| NSC374110 |
| .BETA.-CAROTENE-4,4'-DIONE |
| AKOS040758832 |
| 4,4'-DIKETO-.BETA.-CAROTENE |
| CCG-207976 |
| CS-6879 |
| 2,4,4-trimethyl-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-3-oxocyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohex-2-en-1-one |
| CAS-514-78-3 |
| LS-15425 |
| RO-19915 |
| Canthaxanthin (trans), analytical standard |
| Canthaxanthine 10 microg/mL in Acetonitrile |
| CI-(1975)NO.40850 |
| C08583 |
| AB00053349-02 |
| Q385657 |
| W-105885 |
| 3,3'-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,4,4-trimethylcyclohex-2-enone) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|