| DEHP |
| 117-81-7 |
| Di(2-ethylhexyl)phthalate |
| Di(2-ethylhexyl) phthalate |
| BIS(2-ETHYLHEXYL)PHTHALATE |
| Diethylhexyl phthalate |
| 2-Ethylhexyl phthalate |
| Di-sec-octyl phthalate |
| Octyl phthalate |
| Di-2-ethylhexyl phthalate |
| Fleximel |
| Octoil |
| Ethylhexyl phthalate |
| Palatinol AH |
| Vestinol AH |
| Bisoflex DOP |
| Kodaflex DOP |
| Staflex DOP |
| Truflex DOP |
| Flexol DOP |
| Vinicizer 80 |
| Bisoflex 81 |
| Eviplast 80 |
| Eviplast 81 |
| Hercoflex 260 |
| Etalon |
| RC plasticizer DOP |
| Compound 889 |
| Witcizer 312 |
| Platinol dop |
| Nuoplaz dop |
| Platinol ah |
| Hatcol dop |
| Reomol dop |
| Pittsburgh PX-138 |
| Sansocizer DOP |
| Ergoplast FDO |
| Monocizer DOP |
| Plasthall DOP |
| Flexol plasticizer DOP |
| Mollan O |
| Jayflex DOP |
| Sicol 150 |
| Ergoplast FDO-S |
| Di(2-ethylhexyl)orthophthalate |
| Dioctylphthalate |
| Good-rite gp 264 |
| Reomol D 79P |
| Sconamoll DOP |
| Diacizer DOP |
| Kodaflex DEHP |
| Di(ethylhexyl) phthalate |
| Bis(ethylhexyl) phthalate |
| Rcra waste number U028 |
| Bis(2-ethylhexyl) benzene-1,2-dicarboxylate |
| Phthalic acid dioctyl ester |
| NCI-C52733 |
| Etalon (plasticizer) |
| Di(2-ethylhexyl) o-phthalate |
| Phthalic acid di(2-ethylhexyl) ester |
| Sansocizer R 8000 |
| Caswell No. 392K |
| 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
| Behp |
| Di-2-ethylhexylphthalate |
| DOP |
| DOF [Russian plasticizer] |
| Bis(2-ethylhexyl) 1,2-benzenedicarboxylate |
| CCRIS 237 |
| Di-(2-ethylhexyl) phthalate |
| Bis(2-ethylhexyl) o-phthalate |
| Phthalic acid, bis(2-ethylhexyl) ester |
| Ethyl hexyl phthalate |
| HSDB 339 |
| Di(2-ethylhexyl) orthophthalate |
| DTXSID5020607 |
| CHEBI:17747 |
| EINECS 204-211-0 |
| NSC 17069 |
| Benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
| RCRA waste no. U028 |
| Union carbide flexol 380 |
| EPA Pesticide Chemical Code 295200 |
| Phthalic Acid Bis(2-ethylhexyl) Ester |
| BRN 1890696 |
| UNII-C42K0PH13C |
| 1,2-Benzenedicarboxylic acid bis(2-ethylhexyl) ester |
| Bis-(2-ethylhexyl)ester kyseliny ftalove |
| AI3-04273 |
| C42K0PH13C |
| DAF 68 |
| 50885-87-5 |
| Bis(2-ethylhexyl)Phthalate-13C6 |
| Di(2-ethylhexyl)phthalate (DEHP) |
| Bis-(2-ethylhexyl)ester kyseliny ftalove [Czech] |
| NSC-17069 |
| Phthalic acid bis(2-ethylhexyl ester) |
| NCGC00091499-05 |
| EC 204-211-0 |
| 15495-94-0 |
| 1,2-Benzenedicarboxylic acid, bis-(1-ethylhexyl) ester |
| 14C -DEHP |
| 82208-43-3 |
| 1,2-Benzenedicarboxylic acid, 1,2-bis(2-ethylhexyl) ester |
| Diplast O; ESBO-D 82; Ergoplast FDO; Ergoplast FDO-S; Etalon |
| BIS-(2-ETHYLHEXYL) PHTHALATE |
| Phthalic acid, bis-2-ethylhexyl ester |
| SMR000777878 |
| BIS(2-ETHYLHEXYL) PHTHALATE-D38 |
| Bis-(2-ethylhexyl)ester kyseliny ftalove (czech) |
| Diethylhexylphthalate (Bis-(2-ethylhexyl) Phthalate) |
| ergoplas t fdo |
| octyl ph thalate |
| Palatinol DOP |
| Merrol DOP |
| Palatinol AH-L |
| Hatco DOP |
| Vinycizer 80 |
| 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester, labeled with carbon-14 |
| MFCD00009493 |
| Corflex 400 |
| Di-sec-octylphthalate |
| 352431-42-6 |
| Di-sec octyl phthalate |
| Di-2-ethylhexyphthalate |
| Dioctylphthalate (DOP) |
| DIE (CHRIS Code) |
| EHE (CHRIS Code) |
| Di-sec, octyl phthalate |
| Dioctyl phthalate, 99% |
| bis(2-ethylhexyl)phthalat |
| di-2-ethylhexyl-phthalate |
| 1, bis(ethylhexyl) ester |
| DEHP [MI] |
| Epitope ID:140107 |
| di (2-ethylhexyl)phthalate |
| di-(2-ethylhexyl)phthalate |
| WLN: 8OVR BVO8 |
| bis-(2-ethylhexyl)phthalate |
| Di(2-Ethylhexyl phthalate) |
| PLASTIC ADDITIVE 14 |
| Union carbide flexo l 380 |
| di (2-ethylhexyl) phthalate |
| SCHEMBL20271 |
| Diethylhexylphthalate (DEHP) |
| DIOCTYLPHTHALATE [II] |
| 8033-53-2 |
| bis (2-ethylhexyl) phthalate |
| MLS001333173 |
| MLS001333174 |
| MLS002454397 |
| Bis(2- ethylhexyl) phthalate |
| DTXCID70607 |
| bis (2-Etheylexyl) Phthalate |
| Phtalate de dioctyle secondaire |
| Dioctyl phthalate, >=99.5% |
| CHEMBL1242017 |
| SCHEMBL21733281 |
| HMS2233C15 |
| HMS3374J09 |
| DEHP (Di-2-ethylhexyl phthalate) |
| Di-2-ethylhexyl phthalate (DEHP) |
| AMY40790 |
| Bis(2-ethylhexyl)phthalate (DEHP) |
| HY-B1945 |
| NSC17069 |
| Di (2-ethylhexyl)phthalate (DEHP) |
| Di-(2-ethylhexyl)phthalate (DEHP) |
| Tox21_400084 |
| Bis(2-ethylhexyl)ester phthalic acid |
| DIETHYLHEXYL PHTHALATE [INCI] |
| LS-592 |
| s3360 |
| Bis (2-ethylhexyl)phthalate (DEHP) |
| BIS(2-ETHYLHEXYL PHTHALATE)- |
| Di (2-ethylhexyl) phthalate (DEHP) |
| PHTHALATE, BIS(2-ETHYLHEXYL) |
| AKOS024318875 |
| Di-(2-Ethylhexyl) Phthalate (DEHP) |
| PLASTIC ADDITIVE 14 [USP-RS] |
| NCGC00091499-01 |
| NCGC00091499-02 |
| NCGC00091499-04 |
| NCGC00091499-06 |
| NCGC00091499-07 |
| CAS-117-81-7 |
| DI(2-ETHYLHEXYL)PHTHALATE [IARC] |
| BIS(2-ETHYLHEXYL)PHTHALATE [HSDB] |
| Bis(2-ethylhexyl) 1, 2-benzenedicarboxylate |
| bis-(2-ethylhexyl) 1,2-benzenedicarboxylate |
| CS-0014050 |
| Di-(2-Ethylhexyl) Phthalate (DEHP) Update |
| FT-0624576 |
| FT-0663286 |
| P0297 |
| WLN: 4Y2 & 1OVR BVO1Y4 & 2 |
| bis-(2-ethylhexyl) 1,2-benzenedicar boxylate |
| EN300-93410 |
| PHTHALIC ACID, BIS(2-ETHYLHEXYL)ESTER |
| Bis(2-ethylhexyl) phthalate, Selectophore(TM) |
| C03690 |
| 1,2-bis(2-ethylhexyl) benzene-1,2-dicarboxylate |
| A937603 |
| Bis (2-Ethylhexyl) Phthalate (Dioctyl phthalate) |
| Q418492 |
| 1,2-Benzenedicarboxylic acid bis-(1-ethylhexyl) ester |
| 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl)ester |
| benzene-1,2-dicarboxylic acid bis(2-ethylhexyl) ester |
| BRD-A89471977-001-05-2 |
| Di-sec octyl phthalate (Di-(2-ethylhexyl) phthalate) |
| Bis(2-ethylhexyl) phthalate 100 microg/mL in Methanol |
| Bis(2-ethylhexyl) phthalate 5000 microg/mL in Methanol |
| F0001-0292 |
| Z1267670415 |
| 1,2-Benzenedicarboxylic acid 1,2-bis(2-ethylhexyl) ester |
| 1,2-BENZENEDICARBOXYLIC ACID,BIS(2-ETHYLHEXYLESTER) |
| Bis(2-ethylhexyl) phthalate, SAJ first grade, >=98.0% |
| Di(2-ethylhexyl)phthalate (DEHP), for oral exposures only |
| 1,2-BENZEDICARBOXYLIC ACID, BIS(2-ETHYL-HEXYL) ESTER |
| Bis(2-ethylhexyl) phthalate, PESTANAL(R), analytical standard |
| DI(2-ETHYLHEXYL) PHTHALATE (SEE ALSO CAS 103-23-1) |
| bis(2-ethylhexyl) phthalate; di-(2-ethylhexyl) phthalate; DEHP |
| Di(2-ethylhexyl)phthalate (DEHP), for intravenous exposures only |
| Phthalic acid, bis-2-ethylhexyl ester 10 microg/mL in Cyclohexane |
| Plastic additive 01, European Pharmacopoeia (EP) Reference Standard |
| TRANSGENIC MODEL EVALUATION (DI(2-ETHYLHEXYL) PHTHALATE) |
| Bis(2-ethylhexyl) phthalate, certified reference material, TraceCERT(R) |
| Plastic additive 14, United States Pharmacopeia (USP) Reference Standard |
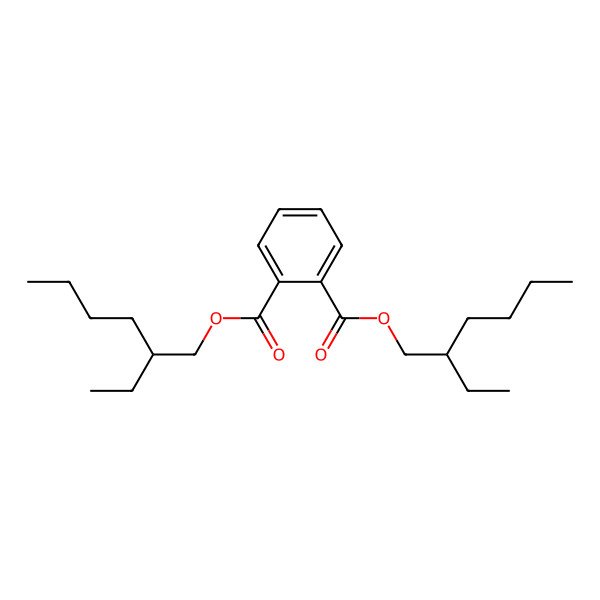
| InChI=1/C24H38O4/c1-5-9-13-19(7-3)17-27-23(25)21-15-11-12-16-22(21)24(26)28-18-20(8-4)14-10-6-2/h11-12,15-16,19-20H,5-10,13-14,17-18H2,1-4H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|