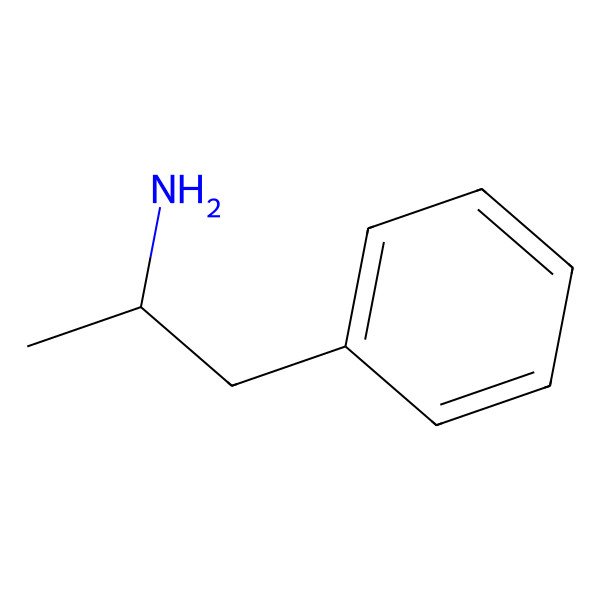
| Amfetamine |
| 1-phenylpropan-2-amine |
| dl-Amphetamine |
| Desoxynorephedrine |
| Norephedrane |
| 1-Phenyl-2-aminopropane |
| 300-62-9 |
| alpha-Methylphenethylamine |
| Elastonon |
| Fenopromin |
| Phenedrine |
| Mydrial |
| beta-Aminopropylbenzene |
| Actedron |
| Adderall |
| Allodene |
| Anorexide |
| Anorexine |
| Benzebar |
| Benzolone |
| Isoamyne |
| Mecodrin |
| Novydrine |
| Oktedrin |
| Ortedrine |
| Percomon |
| Profamina |
| Simpatina |
| Adipan |
| Finam |
| Isomyn |
| 1-Methyl-2-phenylethylamine |
| 1-Phenyl-2-propylamine |
| Protioamphetamine |
| amfetaminum |
| alpha-Methylbenzeneethaneamine |
| 3-Phenyl-2-propylamine |
| Fenylo-izopropylaminyl |
| (Phenylisopropyl)amine |
| dl-alpha-Methylphenethylamine |
| 1-Phenyl-2-propanamine |
| 2-Amino-1-phenylpropane |
| Psychedrine |
| (+-)-Benzedrine |
| racemic-Desoxynor-ephedrine |
| Amfetamin |
| Amfetamina |
| Amphetamin |
| Anfetamina |
| Dyanavel |
| rac-Amphetamine |
| dl-Benzedrine |
| Benzedrine |
| Desoxynorephedrin |
| Propisamine |
| Raphetamine |
| Rhinalator |
| Simpatedrin |
| Sympatedrine |
| beta-Phenylisopropylamin |
| Sympamine |
| Weckamine |
| Dyanavel XR |
| Adzenys ER |
| Amphetamine, dl- |
| 1-Phenyl-2-amino-propan |
| (+-)-alpha-Methylphenylethylamine |
| Amfetamina [Italian] |
| Anfetamina [Spanish] |
| beta-Phenylisopropylamine |
| 60-15-1 |
| Amfetamine [INN:BAN] |
| (+-)-alpha-Methylphenethylamine |
| HSDB 3287 |
| .beta.-Aminopropylbenzene |
| Amfetaminum [INN-Latin] |
| Adderall XR |
| Benzeneethanamine, alpha-methyl-, (+-)- |
| Amfetamina [INN-Spanish] |
| (+-)-alpha-Methylbenzeneethanamine |
| (+/-)-Desoxynorephedrine |
| Benzeneethanamine, .alpha.-methyl- |
| UNII-CK833KGX7E |
| EINECS 200-458-3 |
| EINECS 206-096-2 |
| CK833KGX7E |
| dl-1-Phenyl-2-aminopropane |
| Adzenys XR-ODT |
| NSC 27159 |
| NSC-27159 |
| Phenethylamine, alpha-methyl- |
| beta-Aminopropylbenzene (VAN) |
| .alpha.-Methylbenzeneethanamine |
| DELCOBESE |
| Phenethylamine, alpha-methyl-, (+-)- |
| Norephedrine, deoxy- |
| Fenylo-izopropylaminyl [Polish] |
| 3-AMINO-1-PROPYLBENZENE |
| Benzeneethanamine, alpha-methyl- |
| 1-Phenyl-2-aminopropane (VAN) |
| AI3-02438 |
| beta-Phenylisopropylamin [German] |
| 1-Phenylpropan-2-amin |
| 1-Phenyl-2-amino-propan [German] |
| alpha-Methylphenylethylamine |
| Dexedrine |
| CHEBI:2679 |
| DTXSID4022600 |
| 1-benzylethylamine |
| Amfetamine (INN) |
| NSC27159 |
| NT-0201 |
| AMFETAMINE [INN] |
| beta-phenyl-isopropylamine |
| Phenethylamine, .alpha.-methyl-, (.+/-.)- |
| AMFETAMINE (MART.) |
| AMFETAMINE [MART.] |
| Benzeneethanamine, .alpha.-methyl-, (.+/-.)- |
| Isoamycin |
| DEA No. 1100 |
| (+/-)-1-Phenyl-2-aminopropane |
| Thyramine |
| S(+)-Amphetamine |
| levo-Amphetamine |
| (+/-)-Benzedrine |
| alpha-Methylbenzeneethanamine |
| (+/-)-beta-Phenylisopropylamine |
| amphetaminium |
| Amphetamine Sulfate (2:1) |
| (+)-.alpha.-Methylphenethylamine |
| Adderal |
| Adzenys |
| amphetamine- |
| NSC73713 |
| DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE |
| Amfetamin (TN) |
| Dyanavel (TN) |
| component of Amodex |
| Phenethylamine, d- |
| (+-)-amphetamine |
| Adzenys (TN) |
| (plusmn)-amphetamine |
| Benzeneethanamine, .alpha.-methyl-, (S)- |
| Noclon (Salt/Mix) |
| ACETEDRON |
| Fenamin (Salt/Mix) |
| Ortenal (Salt/Mix) |
| Zedrine (Salt/Mix) |
| Euphodyn (Salt/Mix) |
| Stimulan (Salt/Mix) |
| Fabedrine (Salt/Mix) |
| Oraldrina (Salt/Mix) |
| Vapedrine (Salt/Mix) |
| Sympametin (Salt/Mix) |
| alpha-methylphenethylamin |
| component of Biphetamine |
| Phenethylamine, (+)- |
| 3-phenylpropan-2-amine |
| AMPHETAMINE [MI] |
| (.+/-.)-Benzedrine |
| Benzeneethanamine, (S)- |
| RACEMIC AMPHETAMINE |
| .beta.-Phenylisopropylamin |
| alpha-methyl phenethylamine |
| AMPHETAMINE, (D) |
| Amphetamine; (Benzedrine) |
| (+-)-desoxynor-ephedrine |
| AMPHETAMINE [HSDB] |
| CHEMBL405 |
| D05BMG |
| .beta.-Phenylisopropylamine |
| AMPHETAMINE [VANDF] |
| SCHEMBL8858 |
| .alpha.-Methylphenethylamine |
| AMFETAMINE [WHO-DD] |
| Oprea1_447423 |
| alpha-Methylbenzeneethan-amine |
| d/l-Amphetamine hydrochloride |
| (.+/-.)-Desoxynorephedrine |
| .alpha.-Methylphenylethylamine |
| Bencenoetanamina, Alfa-metil- |
| DivK1c_000991 |
| WLN: ZY1&1R |
| (S)-.alpha.-Phenylethylamine |
| d-.alpha.-Methylphenethylamine |
| DTXCID402600 |
| GTPL4804 |
| WLN: ZY1&1R -D |
| .alpha.-Methylbenzeneethaneamine |
| DL-.alpha.-Methylphenethylamine |
| (+-)-DESOXYNOREPHEDRINE |
| HMS503G03 |
| KBio1_000991 |
| Phenethylamine, .alpha.-methyl- |
| alpha-methyl-beta-phenylethylamine |
| AMPHETAMINE [ORANGE BOOK] |
| N06BA01 |
| (+-)-alpha-methyl phenethylamine |
| (+-)-PHENYLISOPROPYLAMINE |
| (+/-)-alpha-Methylphenethylamine |
| CHEBI:132233 |
| NINDS_000991 |
| rac-(2R)-1-phenylpropan-2-amine |
| (+)-.alpha.-Methylphenylethylamine |
| PHENETHYLAMINE, ALPHA-METHYL |
| RACEMIC DESOXY-NOR-EPHEDRINE |
| .alpha.-Methylphenethylamine, d-form |
| BDBM50005246 |
| (S)-(+)-.beta.-Phenylisopropylamine |
| Phenethylamine, alpha-methyl, (+-)- |
| (+-)-BETA-PHENYLISOPROPYLAMINE |
| (.+/-.)-.beta.-Phenylisopropylamine |
| AB07478 |
| DB00182 |
| LS-1148 |
| LS-1428 |
| (+-)-1-PHENYL-2-AMINOPROPANE |
| (.+/-.)-.alpha.-Methylphenethylamine |
| IDI1_000991 |
| rac-Amphetamine 1.0 mg/ml in Methanol |
| (.+/-.)-.alpha.-Methylphenylethylamine |
| (+-)-ALPHA-METHYLBENZENE-ETHANAMINE |
| (+-)-ALPHA-METHYLPHENYL ETHYLAMINE |
| Benzeneethanamine, alpha-methyl-, (+/-)- |
| (+/-)-.ALPHA.-METHYLPHENETHYLAMINE |
| BENZENEETHANAMINE, ALPHA-METHYL-,(+-) |
| C07514 |
| D03740 |
| D07445 |
| L000864 |
| Q179452 |
| BENZENEETHANAMINE, .ALPHA.-METHYL-, (+/-)- |
| PF-08 (Bio-MD/MPAR/prodrug/oral, ADHD), PharmacoFore |
| SELEGILINE HYDROCHLORIDE IMPURITY B [EP IMPURITY] |
| Amphetamine (tamper and abuse-resistant, Bio-MD/MPAR, ADHD) |
| ALPHA-METHYL-BENZENEETHANAMIDE (SEE ALSO DL-AMPHETAMINE SULFATE) |
| Amphetamine (tamper and abuse-resistant, Bio-MD/MPAR, ADHD), PharmacoFore |
| AMPHETAMINE (SEE ALSO: D-AMPHETAMINE (51-64-9) & AMPHETAMINE SULFATE (60-13-9)) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|