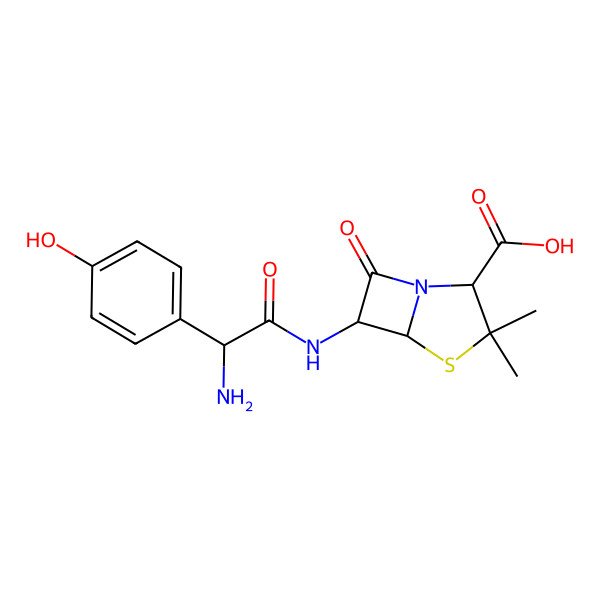
| Amoxycillin |
| 26787-78-0 |
| Amoxicillin anhydrous |
| Amoxicilline |
| p-Hydroxyampicillin |
| Amopenixin |
| Amolin |
| Moxal |
| D-Amoxicillin |
| Amoxil |
| Amoxicillinum |
| Amoxicilina |
| Clamoxyl |
| Amoxiden |
| Amoxivet |
| Anemolin |
| Bristamox |
| Delacillin |
| Flemoxin |
| Hiconcil |
| Histocillin |
| Imacillin |
| Unicillin |
| Vetramox |
| Amoclen |
| Aspenil |
| Cemoxin |
| Efpenix |
| Piramox |
| Amoxi |
| Sumox |
| Amoxi-Mast |
| Sawamox PM |
| Metafarma capsules |
| Metifarma capsules |
| Moxatag |
| Ospamox |
| Amopen |
| Amoxicillin (anhydrous) |
| Amoxicaps |
| Robamox |
| alpha-Amino-p-hydroxybenzylpenicillin |
| Amoxicilina [INN-Spanish] |
| Amoxicilline [INN-French] |
| Amoxicillinum [INN-Latin] |
| Apo-Amoxi |
| AMOXICILLIN PEDIATRIC |
| Amoxycillin Trihydrate |
| BRL-2333 |
| Amoxicillin [INN] |
| 6-(p-Hydroxy-alpha-aminophenylacetamido)penicillanic acid |
| BLP 1410 |
| AMOXICILLIN CRYSTALLINE |
| amoxycilin |
| D-(-)-alpha-Amino-p-hydroxybenzylpenicillin |
| Topramoxin |
| Amoxyke |
| Atoksilin |
| CHEBI:2676 |
| Demoksil |
| Largopen |
| Moksilin |
| Promoxil |
| Remoxil |
| Damoxy |
| HSDB 3204 |
| Ro 10-8756 |
| EINECS 248-003-8 |
| BL-P 1410 |
| D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid |
| Amoxicillin (INN) |
| NSC 277174 |
| NSC-277174 |
| 6-(D-(-)-p-Hydroxy-alpha-aminobenzyl)penicillin |
| AMOX |
| DTXSID3037044 |
| UNII-9EM05410Q9 |
| Amoxicillinum trihydricum |
| Amoxicillin hydrate |
| 6-(D-(-)-alpha-Amino-p-hydroxyphenylacetamido)penicillanic acid |
| 9EM05410Q9 |
| Amoxicillin (as trihydrate) |
| (-)-6-(2-Amino-2-(P-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo-(3.2.0)heptane-2-carboxylic acid |
| (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| (2S,5R,6R)-6-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| DTXCID1017044 |
| EC 248-003-8 |
| Amoksicillin |
| Kentrocyllin |
| Matasedrin |
| Paradroxil |
| Zamocillin |
| Zamocilline |
| Alfamox |
| Amophar |
| Amoxidal |
| Amoxillat |
| Amoxina |
| Amoxine |
| Flemoxine |
| Galenamox |
| Gramidil |
| Himinomax |
| Izoltil |
| Metifarma |
| Pacetocin |
| Pamocil |
| Pasetocin |
| Siganopen |
| Simplamox |
| Sintopen |
| Velamox |
| Amoran |
| Ciblor |
| NSC277174 |
| Amoxi-wolff |
| Uro-clamoxyl |
| Amoxi-Diolan |
| AX |
| Amoksicillin forte |
| A-Gram |
| Dura AX |
| co-amoxiclav (amoxicillin + clavulanic acid) |
| Amoxicillin-ratiopharm |
| AMOXICILLIN (MART.) |
| AMOXICILLIN [MART.] |
| (2S,5R,6R)-6-((R)-2-Amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| (2S,5R,6R)-6-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((2R)-amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-, (2S,5R,6R)- |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-(p-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-, D- |
| 4-Thia-1-azobicyclo(3.2.0)heptane-2-carboxylic acid, 6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-, (2S-(2alpha,5alpha,6beta(S)))- |
| amoxicillanyl |
| Drg-0075 |
| 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carboxylic acid |
| UNII-804826J2HU |
| CHEBI:51254 |
| Amoxicillin (TN) |
| (2S,5R,6R)-6-((R)-(-)-2-AMINO-2-(P-HYDROXYPHENYL)ACETAMIDO)-3,3-DIMETHYL-7-OXO-4-THIA-1-AZABICYCLO(3.2.0)HEPTANE-2-CARBOXYLIC ACID |
| SMR000058707 |
| Amoxicillin (trihydrate) |
| AMOXICILLIN (USP IMPURITY) |
| Amoxycillin (trihydrate) |
| AMOXICILLIN (USP MONOGRAPH) |
| PREVPAC COMPONENT AMOXICILLIN |
| TALICIA COMPONENT AMOXICILLIN |
| Amoxicillin (Amoxycillin) |
| AUGMENTIN COMPONENT AMOXICILLIN |
| AMOXICILLIN TRIHYDRATE (MART.) |
| AMOXICILLIN COMPONENT OF PREVPAC |
| AMOXICILLIN COMPONENT OF TALICIA |
| (2S,5R,6R)-6-(2-Amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| AMOXICILLIN COMPONENT OF AUGMENTIN |
| 61336-70-7 |
| AMOXICILLIN TRIHYDRATE (EP MONOGRAPH) |
| amoxicillina |
| alpha-Amino-p-hydroxybenzylpenicillin trihydrate |
| CLAVULOX COMPONENT AMOXICILLIN TRIHYDRATE |
| AMOXICILLINTRIHYDRATE |
| Amoxi-Inject |
| AMOXICILLIN TRIHYDRATE COMPONENT OF CLAVULOX |
| Amoxi-Tabs |
| notoginsenoside-fe |
| 6beta-((2R)-2-amino-2-(4-hydroxyphenyl)acetamido)-2,2-dimethylpenam-3alpha-carboxylic acid |
| NCGC00016797-02 |
| NCGC00094586-01 |
| (2S,5R,6R)-6-(((2R)-2-amino-2-(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid |
| Prestwick_713 |
| CAS-26787-78-0 |
| Amoxicillinum (Latin) |
| MFCD00056860 |
| AMOXICILLIN [MI] |
| (2S,5R,6R)-6-((R)-(-)-2-AMINO-2-(P-HYDROXYPHENYL)ACETAMIDO)-3,3-DIMETHYL-7-OXO-4-THIA-1-AZABICYCL(3.2.0)HEPTANE-2-CARBOXYLIC ACID TRIHYDRATE |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((2R)-amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-, trihydrate, (2S,5R,6R)- |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-, trihydrate (2S-(2alpha,5alpha,6beta(S*)))- |
| 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-, trihydrate(2S-(2alpha,5alpha,6beta(S*)))- |
| Prestwick0_000357 |
| Prestwick1_000357 |
| Prestwick2_000357 |
| Prestwick3_000357 |
| Epitope ID:114241 |
| Epitope ID:116054 |
| SCHEMBL3427 |
| CHEMBL1082 |
| AMOXICILLIN [WHO-DD] |
| BSPBio_000453 |
| MLS000028632 |
| MLS002222248 |
| SPBio_002374 |
| BPBio1_000499 |
| CHEBI:53712 |
| GTPL10895 |
| HY-B0467A |
| J01CA04 |
| LSQZJLSUYDQPKJ-NJBDSQKTSA-N |
| HMS1569G15 |
| HMS2096G15 |
| HMS2231K23 |
| HMS3259P17 |
| HMS3713G15 |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)- |
| AMOXICILLIN ANHYDROUS [HSDB] |
| Tox21_111302 |
| BDBM50350464 |
| s3015 |
| AKOS025395540 |
| Tox21_111302_1 |
| CCG-220357 |
| DB01060 |
| DS-3835 |
| NC00670 |
| NCGC00179554-01 |
| AC-12263 |
| Amoxicillin, potency: >=900 mug per mg |
| C06827 |
| D07452 |
| EN300-118704 |
| A877139 |
| Q201928 |
| SR-01000721886 |
| SR-01000721886-2 |
| BRD-K55044200-001-03-9 |
| BRD-K55044200-001-15-3 |
| Z1515385072 |
| (2S,5R,6R)-6-((R)-2-Amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid |
| 4-THIA-1-AZABICYCLO(3.2.0)HEPTANE-2-CARBOXYLIC ACID, 6-((AMINO(4-HYDROXYPHENYL)ACETYL)AMINO)-3,3-DIMETHYL-7-OXO-(2S-(2.ALPHA.,5.ALPHA.,6.BETA.(S*)))- |
| 4-Thia-1-Azabicyclo(3.2.0)Heptane-2-Carboxylic Acid, 6-((Amino(4-Hydroxyphenyl)Acetyl)Amino)-3,3-Dimethyl-7-Oxo-(2S-(2Alpha,5Alpha,6Beta(S*)))- |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[amino (4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, [2S-[2.alpha.,5.alpha.,6.beta.(S*)]]- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|