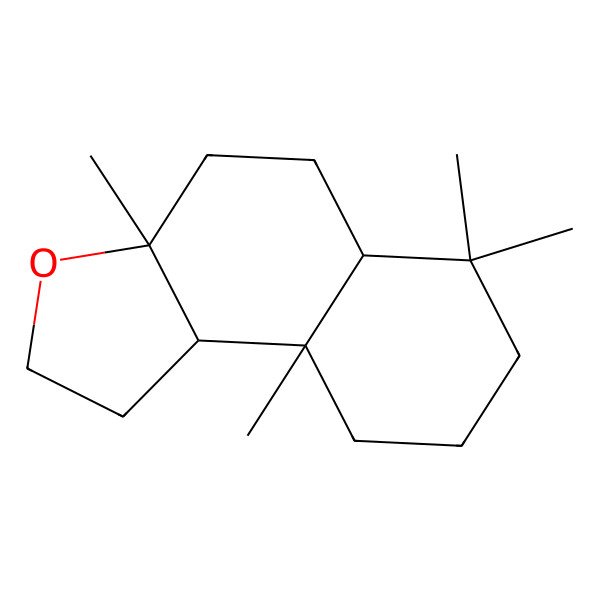
| 6790-58-5 |
| Ambroxide |
| Ambroxan |
| Ambrox |
| (-)-ambrox |
| FEMA No. 3471 |
| (+/-)-Ambrox |
| Ambroxide, (+/-)- |
| K60YJF1E9A |
| TD34B3O8M9 |
| DTXSID0047113 |
| CHEBI:78307 |
| 8alpha,12-Oxido-13,14,15,16-tetranorlabdane |
| (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyldodecahydronaphtho[2,1-b]furan |
| Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)- |
| (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyl-2,4,5,5a,7,8,9,9b-octahydro-1H-benzo[e][1]benzofuran |
| 1,5,5,9-Tetramethyl-13-oxatricyclo (8.3.0.0.(4.9))tridecane |
| 1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(4,9))tridecane, (-)- |
| Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)-rel- |
| 100679-85-4 |
| Ambroxide, (-)- |
| UNII-TD34B3O8M9 |
| 1,5,5,9-Tetramethyl-13-oxatricyclo[8.3.0.04,9]tridecane |
| EINECS 229-861-2 |
| AMBERMOX |
| AMBROFIX |
| ORCANOX |
| AMBROXIDE [MI] |
| UNII-K60YJF1E9A |
| (-)-Ambroxide, 99% |
| EC 229-861-2 |
| (-)-NORLABDANE OXIDE |
| SCHEMBL114912 |
| (-)-Ambroxide, >=99% |
| CHEMBL496447 |
| DTXCID8027113 |
| YPZUZOLGGMJZJO-LQKXBSAESA-N |
| (-)-Ambroxide, analytical standard |
| HY-N1384 |
| Tox21_302674 |
| MFCD00134491 |
| s5175 |
| AKOS025311143 |
| CCG-266845 |
| NCGC00256883-01 |
| AC-35128 |
| AS-56452 |
| Dodecahydro-3a,6,6,9a-tetramethylnaphtho(2,1-b)furan, (3aR-(3aalpha,5abeta,9aalpha,9bbeta))- |
| Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR-(3aalpha,5abeta,9aalpha,9bbeta))- |
| CAS-6790-58-5 |
| CS-0016804 |
| 4-Pentyloxyphenyl-4-Trans-PropylcyclohexylBenzo |
| D88521 |
| A575915 |
| A-575915 |
| Q2793438 |
| F0001-1783 |
| 1,5,5,9-Tetramethyl-13-oxatricyclo[8.3.0.0(4,9)]tridecane |
| 1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(sup 4,9))tridecane |
| (+/-)-(3aalpha,5abeta,9aalpha,9bbeta)-3a,6,6,9a-Tetramethyldodecahydronaphtho(2,1-b)furan |
| Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aalpha,5abeta,9aalpha,9bbeta)- |
| (+/-)-(3A.ALPHA.,5A.BETA.,9A.ALPHA.,9B.BETA.)-3A,6,6,9A-TETRAMETHYLDODECAHYDRONAPHTHO(2,1-B)FURAN |
| (3AR-(3aalpha,5abeta,9aalpha,9bbeta))-dodecahydro-3a,6,6,9a-tetramethylnaphtho(2,1-b)furan |
| NAPHTHO(2,1-B)FURAN, DODECAHYDRO-3A,6,6,9A-TETRAMETHYL-, (3A.ALPHA.,5A.BETA.,9A.ALPHA.,9B.BETA.)- |
| NAPHTHO(2,1-B)FURAN, DODECAHYDRO-3A,6,6,9A-TETRAMETHYL-, (3A.ALPHA.,5A.BETA.,9A.ALPHA.,9B.BETA.)-(+/-)- |
| Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aalpha,5abeta,9aalpha,9bbeta)-(+/-)- |
| NAPHTHO(2,1-B)FURAN, DODECAHYDRO-3A,6,6,9A-TETRAMETHYL-, (3AR-(3A.ALPHA.,5A.BETA.,9A.ALPHA.,9B.BETA.))- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|