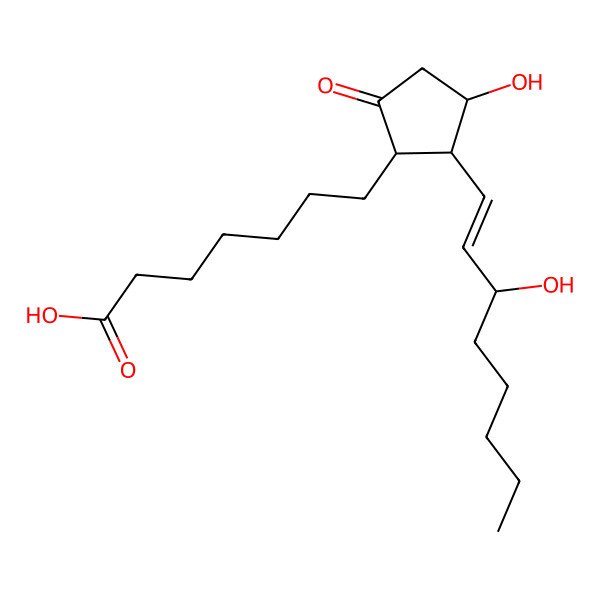
| Prostaglandin E1 |
| 745-65-3 |
| PGE1 |
| Edex |
| Caverject |
| Muse |
| Prostin VR |
| Prostavasin |
| Befar |
| Alprostadilum |
| Femprox |
| Topiglan |
| Alprox-TD |
| Liprostin |
| Prostandin |
| l-Prostaglandin E1 |
| Vitaros |
| Prink |
| Prostin VR Pediatric |
| PGE-1 |
| Lipoprost |
| Promostan |
| Prostivas |
| Caverject Impulse |
| PGE1alpha |
| (-)-Protaglandin E1 |
| (11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid |
| l-PGE1 |
| (13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate |
| 11alpha,15alpha-Dihydroxy-9-oxo-13-trans-prostenoic acid |
| 7-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxyoct-1-en-1-yl)-5-oxocyclopentyl)heptanoic acid |
| U-10136 |
| DTXSID9022578 |
| UNII-F5TD010360 |
| Alprostadil(Caverject) |
| CHEBI:15544 |
| (-)-Prostaglandin E1 |
| ONO 1608 |
| ONO-1608 |
| EINECS 212-017-2 |
| CHEMBL495 |
| Alprostadilum [INN-Latin] |
| F5TD010360 |
| NSC 165559 |
| NSC-165559 |
| U-10,136 |
| AI3-62116 |
| DTXCID502578 |
| Vasaprostan |
| (13E,15S)-11alpha,15-dihydroxy-9-oxoprost-13-en-1-oic acid |
| 9-oxo-11R,15S-dihydroxy-13E-prostaenoic acid |
| U 10136 |
| 7-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]heptanoic acid |
| Alprostadil [USAN:USP:INN:BAN:JAN] |
| Minprog |
| Sugiran |
| Viridal |
| 3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic acid |
| l-3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic acid |
| Alprostadilum (INN-Latin) |
| Prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-, (11alpha,13E,15S)- |
| (1R,2R,3R)-3-Hydroxy-2-((E)-(3S)-3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic acid |
| ALPROSTADIL (MART.) |
| ALPROSTADIL [MART.] |
| ALPROSTADIL (USP-RS) |
| ALPROSTADIL [USP-RS] |
| (13E)-(15S)-11-alpha,15-dihydroxy-9-oxoprost-13-enoate |
| ALPROSTADIL (USP IMPURITY) |
| ALPROSTADIL [USP IMPURITY] |
| Alista |
| ALPROSTADIL (USP MONOGRAPH) |
| ALPROSTADIL [USP MONOGRAPH] |
| FemLife |
| ProstaglandinE1 |
| RayVa |
| Cyclopentaneheptanoic acid, 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-, (-)- |
| Cyclopentaneheptanoic acid, 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-, l- |
| Prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-, (11a,13E,15S)- |
| 7-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)heptanoic acid |
| 7-[(1r,3r)-3-Hydroxy-2-[(1e,3s)-3-Hydroxyoct-1-En-1-Yl]-5-Oxocyclopentyl]heptanoic Acid |
| Alprostadil (USAN:USP:INN:BAN:JAN) |
| SMR000112594 |
| Lipo-PGE1 |
| Befar (TN) |
| Prink (TN) |
| Prostaglandin E1alpha |
| Edex (TN) |
| Muse (TN) |
| SR-01000597593 |
| MR-256 |
| Prostin VR pediatric (TN) |
| Alprostadil Prostoglandin E1 |
| BML1-F06 |
| NSC165559 |
| Alprostadil(usan) |
| (1R,2R,3R)-3-Hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-5-oxocyclopentaneheptanoic acid |
| HEI-507 |
| NCGC00016535-01 |
| 7-((1R,2R,3R)-3-Hydroxy-2-((1E,3S)-3-hydroxyoct-1-en-1-yl)-5-oxocyclopentyl)heptanoic acid |
| 7-[(1R,2R,3R)-3-hydroxy-2-[(1E,3S)-3-hydroxyoct-1-en-1-yl]-5-oxocyclopentyl]heptanoic acid |
| Alprostadil; 7-[(1R,2R,3R)-3-Hydroxy-2-[(1E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]heptanoic acid |
| CAS-745-65-3 |
| MFCD00077860 |
| 11,15-Dihydroxy-9-oxoprost-13-en-1-oic acid |
| PGE1;Prostaglandin E1 |
| Prestwick2_001018 |
| Prestwick3_001018 |
| ALPROSTADIL [INN] |
| ALPROSTADIL [JAN] |
| PROSTINVR PEDIATRIC |
| ALPROSTADIL [USAN] |
| ALPROSTADIL [VANDF] |
| PGE1 (Prostaglandin E1) |
| SCHEMBL33317 |
| ALPROSTADIL [WHO-DD] |
| BSPBio_001175 |
| BSPBio_001488 |
| MLS000758964 |
| MLS001424250 |
| BIDD:GT0747 |
| PROSTAGLANDIN E1 [MI] |
| BPBio1_001293 |
| GTPL1882 |
| Alprostadil (JP17/USP/INN) |
| ALPROSTADIL [ORANGE BOOK] |
| C01EA01 |
| G04BE01 |
| PGE1, Prostaglandin E1, powder |
| HMS1361K10 |
| HMS1571K17 |
| HMS1791K10 |
| HMS1989K10 |
| HMS2052L11 |
| HMS2090L08 |
| HMS2098K17 |
| HMS3268I09 |
| HMS3402K10 |
| HMS3414N09 |
| HMS3648O17 |
| HMS3678N07 |
| HMS3715K17 |
| AMY30076 |
| BCP01740 |
| EX-A1411 |
| HY-B0131 |
| Tox21_110482 |
| 3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic acid |
| BDBM50101853 |
| LMFA03010134 |
| s1508 |
| AKOS015961103 |
| Prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-, (11.alpha.,13E,15S)- |
| AC-6095 |
| BCP9000277 |
| CCG-101188 |
| CS-1905 |
| DB00770 |
| NC00438 |
| IDI1_033958 |
| Prostaglandin E1, >=99.0% (TLC) |
| SMP2_000271 |
| NCGC00025234-02 |
| NCGC00025234-03 |
| NCGC00025234-04 |
| NCGC00025234-05 |
| AS-16360 |
| Prostaglandin E1, PGE1, 745-65-3 |
| Alprostadil 100 microg/mL in Acetonitrile |
| Muse (intraurethral alprostadil suppository) |
| 11,15-Dihydroxy-9-oxoprost-13-en-1-oate |
| AB00514004 |
| P1917 |
| Alprostadil, meets USP testing specifications |
| C04741 |
| C76381 |
| D00180 |
| Prostaglandin E1, >=98% (HPLC), synthetic |
| AB00514004-06 |
| AB00514004-08 |
| AB00514004_09 |
| 11,15-Dihydroxy-9-oxoprost-13-en-1-oic acidl |
| A838163 |
| EN300-22411483 |
| Q579348 |
| SR-01000946253 |
| SR-01000597593-1 |
| SR-01000597593-5 |
| SR-01000597593-6 |
| SR-01000946253-1 |
| W-104416 |
| BRD-K52459643-001-06-0 |
| BRD-K52459643-001-10-2 |
| BRD-K52459643-001-17-7 |
| (13E)-(15S)-11,15-dihydroxy-9-oxoprost-13-enoate |
| (13E)-(15S)-11,15-dihydroxy-9-oxoprost-13-enoic acid |
| Alprostadil, European Pharmacopoeia (EP) Reference Standard |
| (13e)-(15s)-11alpha,15-dihydroxy-9-oxoprost-13-enoic acid |
| (11?,13E,15S)-11,15-Dihydroxy-9-oxo-prost-13-en-1-oic acid |
| (13E)-(15S)-11-alpha,15-dihydroxy-9-oxoprost-13-enoic acid |
| 11,15-dihydroxy-9-oxoprost-13-en-1-oic acid (ACD/Name 4.0) |
| 3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate |
| Alprostadil, United States Pharmacopeia (USP) Reference Standard |
| (+)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-Cyclopentaneheptanoate |
| (+)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-Cyclopentaneheptanoic acid |
| (-)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-Cyclopentaneheptanoate |
| (-)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-Cyclopentaneheptanoic acid |
| (11alpha,12alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid |
| Prostaglandin E1, synthetic, powder, BioReagent, suitable for cell culture |
| Prostaglandin E1, powder, gamma-irradiated, BioXtra, suitable for cell culture |
| XPG |
|
There are more than 10 synonyms. If you wish to see them all click here.
|