| 140-66-9 |
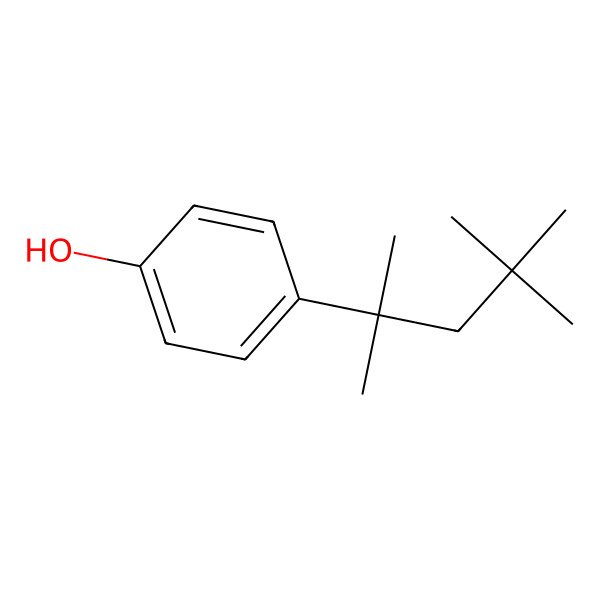
| 4-(2,4,4-trimethylpentan-2-yl)phenol |
| 4-(1,1,3,3-TETRAMETHYLBUTYL)PHENOL |
| 4-t-Octylphenol |
| p-tert-Octylphenol |
| p-Terc.oktylfenol |
| Phenol, 4-(1,1,3,3-tetramethylbutyl)- |
| para-tert-Octylphenol |
| p-(1,1,3,3-Tetramethylbutyl)phenol |
| Phenol, p-(tert-octyl)- |
| p-Octylphenol (VAN) |
| P-T-Octylphenol |
| Phenol, p-(1,1,3,3-tetramethylbutyl)- |
| HSDB 5411 |
| UNII-IOY9FVU3J3 |
| IOY9FVU3J3 |
| p-terc.Oktylfenol [Czech] |
| NSC 5427 |
| EINECS 205-426-2 |
| BRN 0513992 |
| CCRIS 8947 |
| DTXSID9022360 |
| AI3-10011 |
| p-(1',1',3',3'-Tetramethylbutyl)fenol |
| NSC-5427 |
| NSC-7248 |
| tert-Octylphenol, flaked |
| p-(1,1,3,3-tetramethylbutyl)-phenol |
| 4-(TERT-OCTYL)PHENOL |
| OCTYLPHENOL, 4-TERT- |
| CHEMBL259327 |
| DTXCID602360 |
| CHEBI:34445 |
| EC 205-426-2 |
| 4-06-00-03484 (Beilstein Handbook Reference) |
| MFCD00002368 |
| NCGC00164127-02 |
| NCGC00164127-03 |
| p-(1',1',3',3'-Tetramethylbutyl)phenol |
| 4-(2,4,4-TRIMETHYL-2-PENTANYL)PHENOL |
| p-(1,3,3-Tetramethylbutyl)phenol |
| 4-(1,3,3-Tetramethylbutyl)phenol |
| p-(1',3',3'-Tetramethylbutyl)phenol |
| CAS-140-66-9 |
| 4-tertiary-octylphenol |
| WLN: QR DX1 & 1 & 1X1 & 1 & 1 |
| Octylphenol pt |
| 4mga |
| 27L |
| p-tert.-octylphenol |
| 4-tert-octyl-phenol |
| 4-tert.-octylphenol |
| p-(Tert-octyl)-Phenol |
| para-tert.-octyl phenol |
| p-Terc.oktylfenol(Czech) |
| 4-tert-Octylphenol, 97% |
| SCHEMBL10141 |
| BIDD:ER0044 |
| NSC5427 |
| NSC7248 |
| HY-B1941 |
| Tox21_112084 |
| Tox21_400011 |
| BBL027379 |
| BDBM50423506 |
| STK594853 |
| 1,1,3,3-Tetramethyl-4-butylphenol |
| AKOS005516422 |
| Tox21_112084_1 |
| CCG-275197 |
| 1 ,1 ,3,3-Tetramethyl-4-butylphenol |
| 4-(1,1,3,3-tetramethylbutyl) phenol |
| 4-(1,1,3,3-tetramethylbutyl)-phenol |
| 4-tert-Octylphenol, analytical standard |
| NCGC00164127-01 |
| NCGC00164127-04 |
| NCGC00164127-05 |
| NCGC00164127-06 |
| NCGC00164127-07 |
| NCGC00181157-01 |
| 4-(1,1,3,3-tetramethyl-butyl)-phenol |
| Fenol, 4-(1,1,3,3-tetrametilbutil)- |
| VS-08533 |
| LS-105148 |
| Phenol, p- (1,1,3,3-tetramethylbutyl)- |
| CS-0013994 |
| FT-0616437 |
| FT-0673243 |
| p-(1',1',3', 3'-Tetramethylbutyl)phenol |
| Phenol, 4- (1,1,3,3-tetramethylbutyl)- |
| T0144 |
| EN300-20811 |
| E77202 |
| SR-01000944308 |
| J-523827 |
| SR-01000944308-2 |
| W-108198 |
| 4-(1,1,3,3-TETRAMETHYLBUTYL)PHENOL [HSDB] |
| BRD-K64273664-001-01-6 |
| Q15632771 |
| 4-(1,1,3,3-Tetramethylbutyl)phenol (ACD/Name 4.0) |
| Z104483006 |
| 4-tert-Octylphenol, certified reference material, TraceCERT(R) |
| InChI=1/C14H22O/c1-13(2,3)10-14(4,5)11-6-8-12(15)9-7-11/h6-9,15H,10H2,1-5H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|