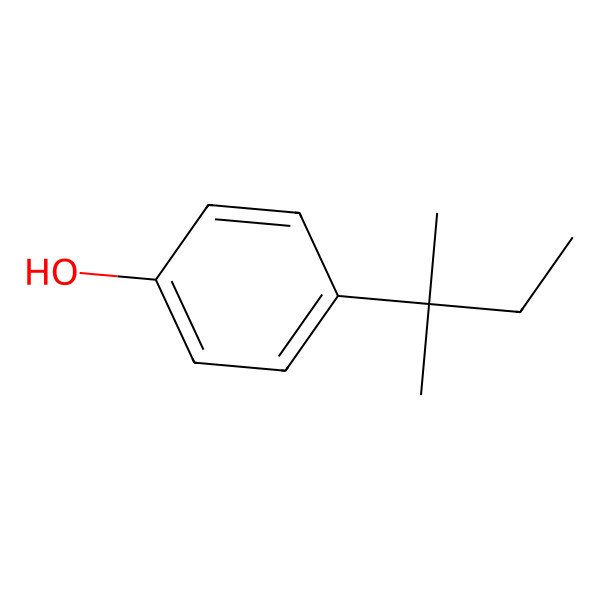
| 80-46-6 |
| 4-tert-Pentylphenol |
| p-tert-Pentylphenol |
| p-tert-Amylphenol |
| 4-t-Amylphenol |
| Pentaphen |
| 4-(2-methylbutan-2-yl)phenol |
| 4-(1,1-DIMETHYLPROPYL)PHENOL |
| Amilphenol |
| Amilfenol |
| Ptap |
| Phenol, 4-(1,1-dimethylpropyl)- |
| Amyl phenol 4T |
| Phenol, p-tert-pentyl- |
| tert-Amylphenol |
| Ucar amyl phenol 4T |
| p-t-Pentylphenol |
| p-(1,1-Dimethylpropyl)phenol |
| 4-(tert-pentyl)phenol |
| 2-Methyl-2-p-hydroxyphenylbutane |
| para-tert-Amylphenol |
| 4-(1,1-Dimethylpropyl)-1-phenol |
| Caswell No. 050 |
| 1-Hydroxy-4-(1,1-dimethylpropyl)benzene |
| 4-t-pentylphenol |
| Phenol, p-(tert-pentyl)- |
| MFCD00002369 |
| NSC 403672 |
| CCRIS 4693 |
| HSDB 5236 |
| EINECS 201-280-9 |
| p-(alpha,alpha-Dimethylpropyl)phenol |
| EPA Pesticide Chemical Code 064101 |
| AMYLPHENOL, P-TERT- |
| BRN 1908224 |
| UNII-6NP9LYK846 |
| AI3-00460 |
| 6NP9LYK846 |
| CHEMBL195693 |
| DTXSID8021771 |
| CHEBI:35096 |
| NSC-403672 |
| NCGC00091655-02 |
| p-(.alpha.,.alpha.-Dimethylpropyl)phenol |
| Phenol,1-dimethylpropyl)- |
| EC 201-280-9 |
| 4-06-00-03383 (Beilstein Handbook Reference) |
| DTXCID001771 |
| WLN: QR D1X1&1&1 |
| p-t-amylphenol |
| CAS-80-46-6 |
| p-(tert-Amyl)phenol |
| p-tertamylphenol |
| p-t-Amyl phenol |
| Nipacide PTAP |
| Pentaphen 67 |
| Para-tertiary amylphenol |
| 4-tert-Amylphenol, 99% |
| AMYLPHENOL [MART.] |
| SCHEMBL49704 |
| MLS002152935 |
| BIDD:ER0210 |
| NRZWYNLTFLDQQX-UHFFFAOYSA- |
| NSC4965 |
| P-TERT-PENTYLPHENOL [MI] |
| p-(1,1-Dimethyl propyl) phenol |
| 4-(1,1-Dimethyl-propyl)-phenol |
| Fenol, 4-(1,1-dimetilpropil)- |
| HMS3039M10 |
| NSC-4965 |
| Tox21_111159 |
| Tox21_202351 |
| Tox21_300088 |
| BDBM50410536 |
| NSC403672 |
| AKOS000119604 |
| Tox21_111159_1 |
| 4-tert-Amylphenol, analytical standard |
| LS-1796 |
| 1-Hydroxy-4-(2-methyl-2-butyl)benzene |
| NCGC00091655-01 |
| NCGC00091655-03 |
| NCGC00091655-04 |
| NCGC00091655-05 |
| NCGC00254041-01 |
| NCGC00259900-01 |
| AC-16506 |
| SMR001224530 |
| A0460 |
| AM20041188 |
| CS-0152629 |
| FT-0704191 |
| 4-(1,1-DIMETHYLPROPYL)PHENOL [HSDB] |
| EN300-20303 |
| A24574 |
| E76129 |
| P-(1,1-Dimethylpropyl)phenoL;Para-tert-amylphenol |
| W-109280 |
| Q26840951 |
| Z104477686 |
| InChI=1/C11H16O/c1-4-11(2,3)9-5-7-10(12)8-6-9/h5-8,12H,4H2,1-3H3 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|