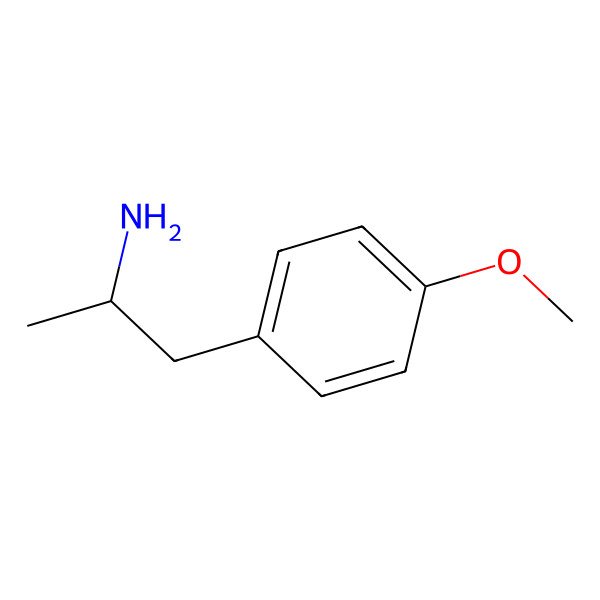
| p-Methoxyamphetamine |
| 1-(4-methoxyphenyl)propan-2-amine |
| para-methoxyamphetamine |
| 64-13-1 |
| 1-(4-Methoxybenzyl)ethylamine |
| paramethoxyamphetamine |
| beta-methoxyamphetamine |
| 1-p-Methoxyphenyl-2-propylamine |
| 1-p-Methoxyphenyl-2-aminopropane |
| 2-Amino-1-(4'-methoxyphenyl)propane |
| (+-)-p-Methoxyamphetamine |
| p-Methoxy-alpha-methylphenethylamine |
| P-methoxyamfetamine |
| 4-methoxyamfetamine |
| DEA No. 7411 |
| 23239-32-9 |
| Death [Street Name] |
| 2-(4-Methoxy-phenyl)-1-methyl-ethylamine |
| D,L-p-methoxyamphetamine |
| NSC 32757 |
| UNII-OVB8F8P39Q |
| DL-p-Methoxy-alpha-methylphenethylamine |
| (+-)-4-Methoxyamphetamine |
| OVB8F8P39Q |
| benzeneethanamine, 4-methoxy-alpha-methyl- |
| Chicken Powder [Street Name] |
| Chicken Yellow [Street Name] |
| (+-)-1-(p-Methoxyphenyl)-2-aminopropane |
| CHEMBL278663 |
| alpha-Methyl-beta-(p-methoxyphenyl)ethylamine |
| HSDB 7594 |
| alpha-methyl-4-methoxyphenethylamine |
| EINECS 200-577-0 |
| NSC-32757 |
| 2-(4-Methoxyphenyl)-1-methylethylamine |
| (+-)-p-Methoxy-alpha-methylphenethylamine |
| Phenethylamine, p-methoxy-.alpha.-methyl- |
| (+-)-p-Methoxy-alpha-methylphenylethylamine |
| (2RS)-1-(4-methoxyphenyl)propan-2-amine |
| (+-)-4-Methoxy-alpha-methylbenzeneethanamine |
| Benzeneethanamine, 4-methoxy-.alpha.-methyl- |
| .alpha.-Methyl-.beta.-(p-methoxyphenyl)ethylamine |
| Benzenethanamine,4-methoxy-.alpha.-methyl-(.+/-.)- |
| Phenethylamine, p-methoxy-.alpha.-methyl-, (.+/-.)- |
| 4-methoxyamphetamine, (+-)-isomer |
| DEATH |
| Formoterol Impurity G |
| DR. DEATH |
| PMA (PSYCHEDELIC) |
| chloroprednisone21-acetate |
| D0B5OI |
| D0R7ZC |
| (d,l)-4-Methoxyamphetamine |
| (+/-)-p-Methoxyamphetamine |
| 1-(4-methoxybenzyl)ethylamin |
| (+/-)-4-Methoxyamphetamine |
| SCHEMBL264634 |
| SCHEMBL721008 |
| Formoterol Fumarate Impurity G |
| (.+/-.)-p-Methoxyamphetamine |
| DTXSID7040578 |
| (.+/-.)-4-Methoxyamphetamine |
| NSC32757 |
| 1-(4-Methoxyphenyl)-2-propanamine |
| 4-Methoxy-alpha-methylphenethylamine |
| 4-METHOXYAMFETAMINE [MART.] |
| 1-(4-methoxyphenyl)-2-aminopropane |
| BDBM50024209 |
| STK664902 |
| AKOS005536031 |
| p-Methoxy-.alpha.-methylphenethylamine |
| 4-Methoxy-alpha-methylbenzeneethanamine |
| AB51426 |
| AB70588 |
| CS-O-31109 |
| DB01472 |
| MB01555 |
| Phenethylamine, p-methoxy-alpha-methyl- |
| NCGC00168268-01 |
| DL-p-Methoxy-.alpha.-methylphenethylamine |
| NCI60_002842 |
| J213.549C |
| J434.573H |
| LS-103576 |
| (+/-)-1-(p-Methoxyphenyl)-2-aminopropane |
| (-)-2-(p-methoxyphenyl)-1-methylethylamine |
| FT-0690314 |
| N-(2-(p-methoxyphenyl)-1-methylethyl)amine |
| (+/-)-p-Methoxy-alpha-methylphenylethylamine |
| [2-(4-methoxyphenyl)-1-methyl-ethyl]-amine |
| (-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine |
| (.+/-.)-1-(p-Methoxyphenyl)-2-aminopropane |
| (.+/-.)-p-Methoxy-.alpha.-methylphenethylamine |
| 2-(4-Methoxy-phenyl)-1-methyl-ethylamine(PMA) |
| (+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine |
| Benzeneethanamine, 4-methoxy-alpha-methyl- (9CI) |
| Benzeneethanamine, 4-methoxy-alpha-methyl-, (+)- |
| Q230005 |
| (R)-(-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine |
| (S)-(+)2-(4-Methoxy-phenyl)-1-methyl-ethylamine |
| Benzeneethanamine, 4-methoxy-alpha-methyl-, (+-)- |
| (1S)-2-(4-METHOXYPHENYL)-1-METHYLETHYLAMINE |
| PHENETHYLAMINE, p-METHOXY-alpha-METHYL-, (+-)- |
| Phenethylamine, p-methoxy-alpha-methyl- (6CI,7CI,8CI) |
| Benzeneethanamine, 4-methoxy-.alpha.-methyl-, (.+/-.)- |
| FORMOTEROL FUMARATE DIHYDRATE IMPURITY G [EP IMPURITY] |
|
There are more than 10 synonyms. If you wish to see them all click here.
|