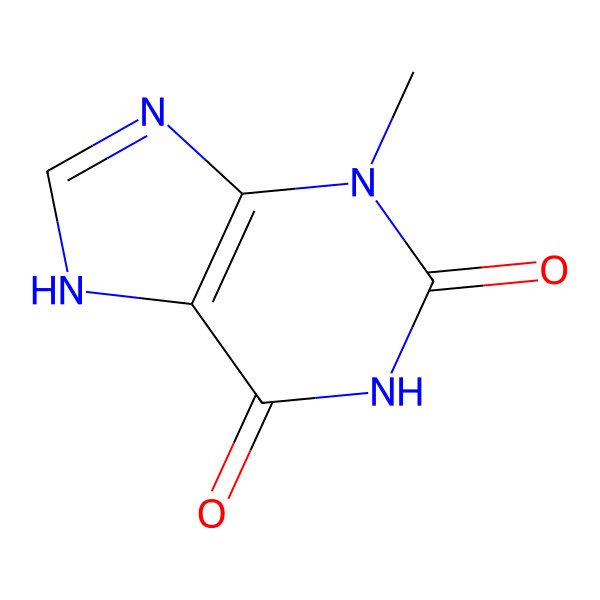
| 1076-22-8 |
| 2,6-Dihydroxy-3-methylpurine |
| 3-Methyl-1H-purine-2,6(3H,7H)-dione |
| 3-methyl-7H-purine-2,6-dione |
| 3 MX |
| Xanthine, 3-methyl- |
| 3-METHYL XANTHINE |
| 1H-Purine-2,6-dione, 3,7-dihydro-3-methyl- |
| 3-methyl-7H-xanthine |
| 3,7-Dihydro-3-methyl-1H-purine-2,6-dione |
| CCRIS 5817 |
| 3-methyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione |
| EINECS 214-058-1 |
| MFCD00005580 |
| CHEMBL619 |
| UNII-WS6X982OEC |
| NSC 515466 |
| WS6X982OEC |
| 3-methyl-3,7-dihydro-1H-purine-2,6-dione |
| CHEBI:62207 |
| NSC-515466 |
| 3-Methyl-3,9-dihydro-purine-2,6-dione |
| 3-Methyl-3,9-dihydro-1H-purine-2,6-dione |
| 3-Methyl-3,7-dihydro-1H-purine-2,6-dione (3-Methylxanthine) |
| 3-methyl-3,7(9)-dihydro-purine-2,6-dione |
| 3-methyl-xanthine |
| 3-methyl-9H-xanthine |
| Spectrum_001898 |
| 3-methyl-1H-purine-2,6(3H,9H)-dione |
| SpecPlus_000737 |
| Linagliptin Intermediates |
| Spectrum2_000502 |
| Spectrum3_001652 |
| Spectrum4_001571 |
| Spectrum5_001544 |
| 3-Methylxanthine, 98% |
| N(1)-demethyltheophylline |
| 2-oxo-3-methylhypoxanthine |
| Oprea1_233226 |
| Oprea1_288071 |
| BSPBio_003403 |
| KBioGR_002122 |
| KBioSS_002428 |
| BIDD:GT0266 |
| DivK1c_006833 |
| SCHEMBL237146 |
| SPECTRUM1504182 |
| SPBio_000423 |
| SCHEMBL8663339 |
| 3-Methyl-3H-purine-2,6-diol |
| 3-methyl-9H-purine-2,6-dione |
| CHEBI:62208 |
| KBio1_001777 |
| KBio2_002422 |
| KBio2_004990 |
| KBio2_007558 |
| KBio3_002623 |
| DTXSID90148107 |
| 1-METHYLXANTHINE (1-MX) |
| BCP18161 |
| BBL012772 |
| BDBM50001515 |
| CCG-39565 |
| GEO-01981 |
| NSC515466 |
| s6186 |
| STK776266 |
| WLN: T56 BM DN FNVMVJ F1 |
| AKOS002272340 |
| AKOS004120009 |
| AKOS006221835 |
| CS-W020049 |
| DS-1280 |
| 3-methyl-3,7-dihydropurine-2,6-dione |
| 1H-Purine-2, 3,7-dihydro-3-methyl- |
| NCGC00095330-01 |
| NCGC00095330-02 |
| NCGC00178088-01 |
| 3-Methyl-3,7-dihydro-purine-2,6-dione |
| HY-50723 |
| PD001242 |
| SY031706 |
| LS-162544 |
| 3,9-dihydro-3-methyl-1H-purine-2,6-dione |
| AM20080030 |
| FT-0616197 |
| M2073 |
| THEOPHYLLINE IMPURITY B [EP IMPURITY] |
| EN300-212022 |
| PENTOXIFYLLINE IMPURITY B [EP IMPURITY] |
| 3-Methyl-3,9-dihydro-1H-purine-2,6-dione # |
| 3-methyl-3,9-dihydro-2H,6H-purine-2,6-dione |
| 3-methyl-7H-purine-2,6-dione;3-Methylxanthine |
| A801730 |
| J-505016 |
| W-108741 |
| Q27888118 |
| THEOPHYLLINE MONOHYDRATE IMPURITY B [EP IMPURITY] |
| Z1741977122 |
| 3-Methyl-3,9-dihydro-purine-2,6-dione(3-methyl xanthine) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|