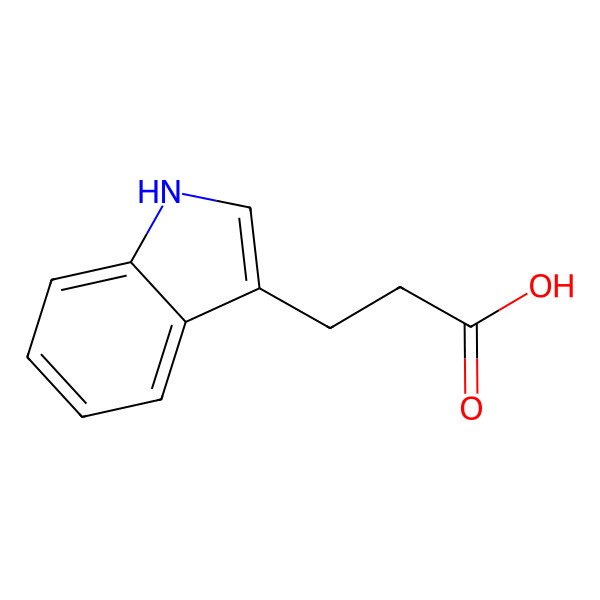
| 830-96-6 |
| Indole-3-propionic acid |
| 3-(1H-Indol-3-yl)propanoic acid |
| 1H-Indole-3-propanoic acid |
| Indolepropionic acid |
| 3-(3-Indolyl)propionic acid |
| 3-(3-Indolyl)propanoic acid |
| Indolylpropionic Acid |
| Oxigon |
| IPA (auxin) |
| 1H-INDOLE-3-PROPIONIC ACID |
| 3-(Indol-3-yl)propionic acid |
| 3-(3-Indole)propionic acid |
| 3-(2-Carboxyethyl)-1H-indole |
| 3-Indolepropionicacid |
| beta-Indolylpropionate |
| beta-(3-Indolyl)propionic acid |
| MFCD00005660 |
| 3-(Indol-3-yl)propanoic acid |
| indole-3-propanoic acid |
| 3-(1H-Indol-3-yl)propionic acid |
| NSC 3252 |
| NSC 47831 |
| 3-(1H-indol-3-yl)-propionic acid |
| .beta.-Indolepropionic acid |
| JF49U1Q7KN |
| VP-20629 |
| .beta.-Indole-3-propionic acid |
| CHEBI:43580 |
| .beta.-(3-Indolyl)propionic acid |
| NSC-3252 |
| NSC-47831 |
| .beta.-Indolylpropionate |
| 3-(1H-Indol-3-yl)propionate |
| 1H-Indole-3-proponoic acid |
| CCRIS 4424 |
| EINECS 212-600-1 |
| UNII-JF49U1Q7KN |
| BRN 0147733 |
| AI3-17433 |
| b-Indolepropionate |
| 4ejl |
| 3-Indolepropionate |
| Indole-3-propionic |
| beta-Indolepropionate |
| 3-indolpropionic acid |
| b-Indolepropionic acid |
| b-Indole-3-propionate |
| 3-Indolepropenoic Acid |
| Acid, 7 |
| VP 20629 |
| 3-Indole propionic acid |
| 1H-Indole-3-propionate |
| beta-Indolepropionic acid |
| indole-3-proprionic acid |
| b-(3-Indolyl)propionate |
| beta-Indole-3-propionate |
| Maybridge1_002431 |
| 3-(3-Indolyl)propanoate |
| 3-(3-Indolyl)propionate |
| b-Indole-3-propionic acid |
| beta-(3-Indolyl)propionate |
| Oprea1_071255 |
| b-(3-Indolyl)propionic acid |
| beta-Indole-3-propionic acid |
| Trihy(droxymethyl)aminomethane |
| 5-22-03-00114 (Beilstein Handbook Reference) |
| BIDD:GT0788 |
| DivK1c_001183 |
| SCHEMBL195405 |
| 3-(3-indolyl)-propionic acid |
| CHEMBL207225 |
| GTPL4709 |
| SCHEMBL1767754 |
| SHP622 |
| DTXSID7061192 |
| BDBM31879 |
| HMS548G11 |
| NSC3252 |
| SHP-622 |
| HMS3604I20 |
| HMS3886E03 |
| BCP26573 |
| NSC47831 |
| s4809 |
| 3-(1H-Indol-3-yl)propanoic acid # |
| AKOS000120911 |
| CCG-115418 |
| CS-W015945 |
| DB02758 |
| FS-3158 |
| HY-W015229 |
| SDCCGMLS-0065895.P001 |
| VP20629 |
| CDS1_000143 |
| NCGC00340705-01 |
| AC-10570 |
| BP-21044 |
| SY015400 |
| Indole-3-propionic acid, >=97.0% (T) |
| Indole-3-propionic acid, >=99.0% (T) |
| AM20060737 |
| FT-0627221 |
| I0032 |
| EN300-20789 |
| 3-Indolepropionic acid, ReagentPlus(R), 99% |
| A15833 |
| C22236 |
| I-4000 |
| AB01333057-02 |
| J-650104 |
| Q21098993 |
| 3-Indolepropionic acid, Vetec(TM) reagent grade, 99% |
| F0849-0388 |
| Z104482518 |
| 10265-77-7 |
| InChI=1/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|