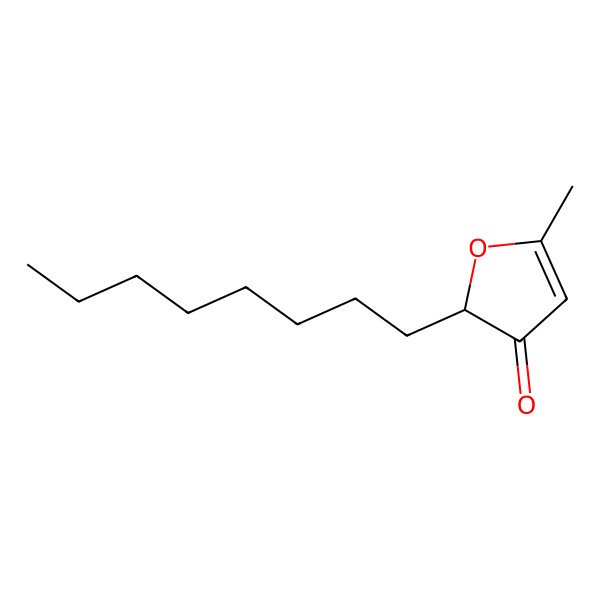
(2R)-5-methyl-2-octylfuran-3-one
| Internal ID | bd007f31-89b3-4d7e-b022-2cfca356f536 |
| Taxonomy | Organoheterocyclic compounds > Dihydrofurans > Furanones |
| IUPAC Name | (2R)-5-methyl-2-octylfuran-3-one |
| SMILES (Canonical) | CCCCCCCCC1C(=O)C=C(O1)C |
| SMILES (Isomeric) | CCCCCCCC[C@@H]1C(=O)C=C(O1)C |
| InChI | InChI=1S/C13H22O2/c1-3-4-5-6-7-8-9-13-12(14)10-11(2)15-13/h10,13H,3-9H2,1-2H3/t13-/m1/s1 |
| InChI Key | ZLLDSWALGJWTSW-CYBMUJFWSA-N |
| Popularity | 0 references in papers |
| Molecular Formula | C13H22O2 |
| Molecular Weight | 210.31 g/mol |
| Exact Mass | 210.161979940 g/mol |
| Topological Polar Surface Area (TPSA) | 26.30 Ų |
| XlogP | 4.40 |
| There are no found synonyms. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL230 | P35354 | Cyclooxygenase-2 | 96.15% | 89.63% |
| CHEMBL2581 | P07339 | Cathepsin D | 93.24% | 98.95% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 87.91% | 95.56% |
| CHEMBL3401 | O75469 | Pregnane X receptor | 87.35% | 94.73% |
| CHEMBL2265 | P23141 | Acyl coenzyme A:cholesterol acyltransferase | 86.80% | 85.94% |
| CHEMBL4769 | O95749 | Geranylgeranyl pyrophosphate synthetase | 86.62% | 92.08% |
| CHEMBL4481 | P35228 | Nitric oxide synthase, inducible | 86.11% | 94.80% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 85.91% | 99.17% |
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 85.08% | 97.25% |
| CHEMBL3038477 | P67870 | Casein kinase II alpha/beta | 82.03% | 99.23% |
| CHEMBL2996 | Q05655 | Protein kinase C delta | 81.87% | 97.79% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| Allium fistulosum |
| PubChem | 162966106 |
| LOTUS | LTS0223326 |
| wikiData | Q105378959 |