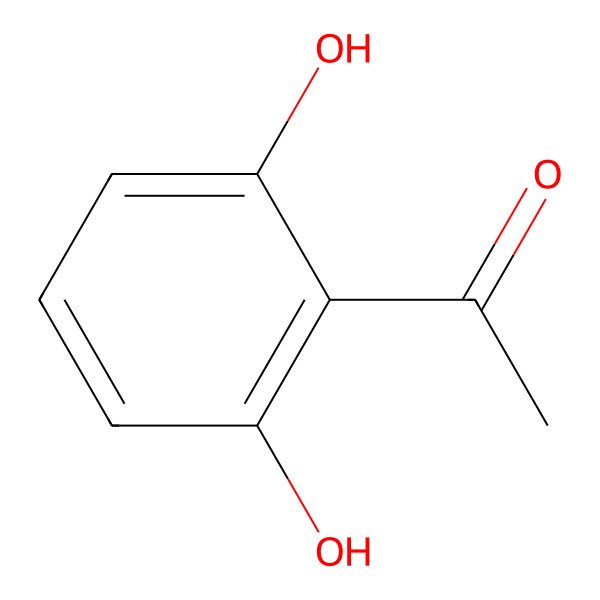
| 699-83-2 |
| 1-(2,6-Dihydroxyphenyl)ethanone |
| 2,6-Dihydroxyacetophenone |
| 2-Acetylresorcinol |
| Resorcinol, 2-acetyl- |
| 1-(2,6-dihydroxyphenyl)ethan-1-one |
| Ethanone, 1-(2,6-dihydroxyphenyl)- |
| gamma-Resacetophenone |
| MFCD00002270 |
| 1,3-Benzenediol, 2-acetyl- |
| .gamma.-Resacetophenone |
| Acetophenone, 2',6'-dihydroxy- |
| 2,6-dihydroxy acetophenone |
| NSC 615 |
| 2, 6-Dihydroxyacetophenone |
| Acetyl-2,6-dihydroxybenzene |
| 2',6'-dihydroxy-acetophenone |
| UNII-88BO51G3Y2 |
| CHEMBL454739 |
| 2-Acetyl-1,3-dihydroxybenzene |
| NSC-615 |
| 88BO51G3Y2 |
| 1-(2,6-dihydroxyphenyl)-ethanone |
| EINECS 211-833-6 |
| 1-(2,6-dihydroxy-phenyl)-ethanone |
| 1-[2,6-bis(oxidanyl)phenyl]ethanone |
| EC 211-833-6 |
| 1-(2,6-Dihydroxyphenyl)ethanone (2',6'-Dihydroxyacetophenone) |
| 2-Acetyl-Resorcinol |
| mono acetyl resorcinol |
| 2-ACETYL RESORCINOL |
| Acetophenone,6'-dihydroxy- |
| 2-Acetyl-1,3-Benzenediol |
| 2`,6`-Dihydroxyacetophenone |
| Ethanone,6-dihydroxyphenyl)- |
| BIDD:ER0607 |
| SCHEMBL105807 |
| NSC615 |
| (2',6'-dihydroxy)acetophenone |
| 2'',6''-dihydroxyacetophenone |
| 2',-6'-dihydroxy-acetophenone |
| 2/',6/'-Dihydroxyacetophenone |
| 2\',6\'-Dihydroxyacetophenone |
| laquo gammaRaquo -resacetophenone |
| YPTJKHVBDCRKNF-UHFFFAOYSA- |
| DTXSID00220185 |
| CHEBI:173644 |
| AMY21894 |
| HY-Y0106 |
| 2',6'-Dihydroxyacetophenone, 97% |
| BDBM50249071 |
| CK2557 |
| STL195537 |
| ACETOPHENONE, 2,6-DIHYDROXY- |
| AKOS000299253 |
| CS-T-19055 |
| CS-W019984 |
| FS-2546 |
| LS-3428 |
| 1-(2,6-Dihydroxyphenyl)ethanone, 9CI |
| NCGC00166008-01 |
| AC-10724 |
| Acetophenone, 2',6'-dihydroxy- (8CI) |
| PD158440 |
| SY001633 |
| D1716 |
| FT-0610665 |
| Ethanone, 1-(2,6-dihydroxyphenyl)- (9CI) |
| D-3400 |
| EN300-114470 |
| A836723 |
| AB-131/40185723 |
| SODIUM CROMOGLICATE IMPURITY A [EP IMPURITY] |
| W-203531 |
| Q27269919 |
| Z1255485184 |
| 2',6'-Dihydroxyacetophenone, matrix substance for MALDI-MS, >=99.5% |
| 2 inverted exclamation mark ,6 inverted exclamation mark -Dihydroxyacetophenone |
| 2',6'-Dihydroxyacetophenone, matrix substance for MALDI-MS, >=99.5% (HPLC), Ultra pure |
| InChI=1/C8H8O3/c1-5(9)8-6(10)3-2-4-7(8)11/h2-4,10-11H,1H3 |
| 9EQ |
|
There are more than 10 synonyms. If you wish to see them all click here.
|