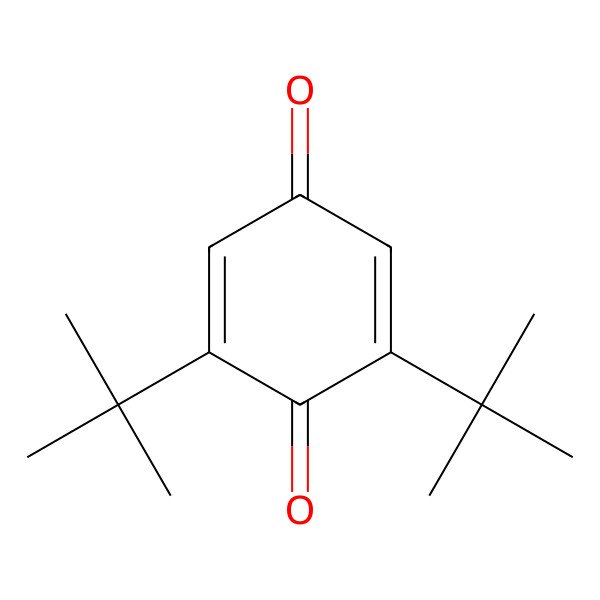
| 2,6-Di-tert-butyl-P-benzoquinone |
| 2,6-Di-tert-butylcyclohexa-2,5-diene-1,4-dione |
| 2,6-Di-tert-butyl-1,4-benzoquinone |
| 2,6-Di-tert-butylbenzoquinone |
| 2,6-Di-tert-butylquinone |
| 2,6-DI-T-BUTYL-P-BENZOQUINONE |
| 2,5-Cyclohexadiene-1,4-dione, 2,6-bis(1,1-dimethylethyl)- |
| p-Benzoquinone, 2,6-di-tert-butyl- |
| 2,6-Bis(1,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione |
| 2,6-Di-t-butyl-1,4-benzoquinone |
| NSC 14448 |
| 2,6-di(tert-butyl)benzo-1,4-quinone |
| 2,6-ditert-butylcyclohexa-2,5-diene-1,4-dione |
| CCRIS 7070 |
| DBQ |
| HSDB 2775 |
| EINECS 211-946-0 |
| UNII-4C9D8L0Y0T |
| AI3-61049 |
| 4C9D8L0Y0T |
| 2,6-bis-tert-Butylbenzoquinone |
| DTXSID7021493 |
| CHEBI:89187 |
| 2,6-di-tert-Butyl-para-benzoquinone |
| NSC-14448 |
| 2,6-di-tert-butylbenzo-1,4-quinone |
| Benzoquinone, 2,6-di-(1,1-dimethylethyl) |
| p-Benzoquinone, 2,6-bis-(1,1-dimethylethyl) |
| 2,5-Cyclohexadien-1,4-dione, 2,6-bis(1,1-dimethylethyl)- |
| 2,6-bis-(1,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione |
| 2,6-Ditert-butylbenzo-1,4-quinone |
| Di-t-butyl benzoquinone |
| Maybridge1_002204 |
| CBMicro_047944 |
| 3,5-di-tert-butylquinone |
| 2, 6-Di-tert-butylquinone |
| SCHEMBL853674 |
| 2,6-ditert.butyl-benzoquinone |
| 2,6-Di-tert-butyl-p-quinone |
| DTXCID801493 |
| CHEMBL3185121 |
| HMS547M04 |
| p-Benzoquinone,6-di-tert-butyl- |
| AMY39482 |
| NSC14448 |
| 2, 6-Di-tert-butyl-p-benzoquinone |
| Tox21_200521 |
| BTB 09891 |
| CCG-49871 |
| MFCD00001601 |
| 2,6-di(tertiarybutyl)-p-benzoquinone |
| 2,6-di-tertiary-butyl-p-benzoquinone |
| 2,6-ditert-butyl-[1,4]benzoquinone |
| 3,5-di-tertiary-butyl-p-benzoquinone |
| AKOS015841128 |
| 2,6-di(tertiary-butyl)-p-benzoquinone |
| CS-W013138 |
| 2,6-Di-tert-butyl-[1,4]benzoquinone |
| 2,6-Di-tert.-butyl-1,4-benzoquinone |
| 2,6-Ditert-butylbenzo-1,4-quinone # |
| NCGC00248670-01 |
| NCGC00258075-01 |
| 3,5-DI-TERT-BUTYL-P-BENZOQUINONE |
| AS-18524 |
| CAS-719-22-2 |
| LS-40333 |
| BIM-0047946.P001 |
| 2,4-dione, 2,6-bis(1,1-dimethylethyl)- |
| 2,6-bis(1,1-dimethylethyl)-p-benzoquinone |
| 2,6-Di-tert-butyl-1,4-benzoquinone, 98% |
| D2256 |
| FT-0610728 |
| 2,6-DI-T-BUTYL-P-BENZOQUINONE [HSDB] |
| D90022 |
| 2,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione |
| A837359 |
| J-507389 |
| SR-01000639296-1 |
| 2,6-Ditert-butylbenzo-1,4-quinone (ACD/Name 4.0) |
| Q27161373 |
| 2,6-Bis-(1,1-Dimethylethyl)-2,5-cycloxehadien-1,4-dione |
| 2,6-Bis(1, 1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione |
| BIS(1,1-DIMETHYLETHYL)-2,6-CYCLOHEXADIENE-1,4-DIONE, 2,6- |
| InChI=1/C14H20O2/c1-13(2,3)10-7-9(15)8-11(12(10)16)14(4,5)6/h7-8H,1-6H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|