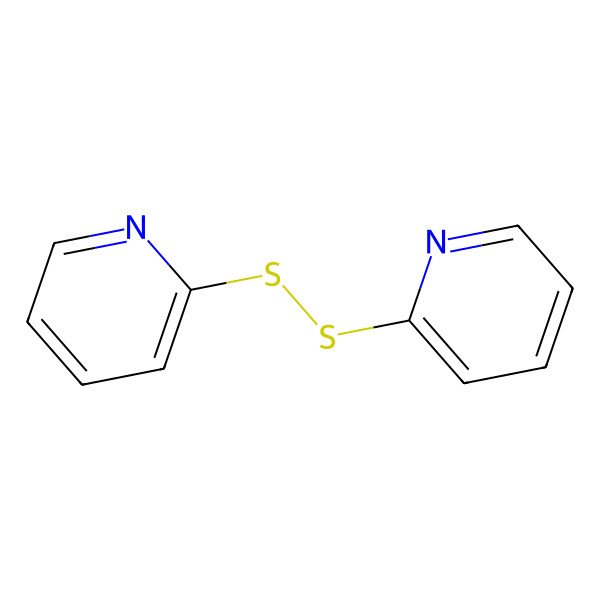
| 2,2'-Dipyridyl disulfide |
| 2127-03-9 |
| 1,2-di(pyridin-2-yl)disulfane |
| Aldrithiol-2 |
| 2-(pyridin-2-yldisulfanyl)pyridine |
| Aldrithiol 2 |
| 2,2'-Dipyridyldisulfide |
| 2-Dipyridyl disulfide |
| 2-Pyridinyl disulfide |
| Bis(2-pyridyl) disulfide |
| Pyridine, 2,2'-dithiobis- |
| 2,2'-Dithiopyridine |
| 2-(2-pyridyldisulfanyl)pyridine |
| 2PDS |
| 2,2'-pyridine disulfide |
| Bis(2-pyridinyl) disulfide |
| Bis(pyridin-2-yl) disulfide |
| 2-Pyridyl disulfide |
| OPSS |
| 2,2-Dithiodipyridine" |
| Di-2-Pyridyl disulfide |
| bis(2-pyridyl)disulfide |
| 2,2'-Dithiobispyridine |
| NSC-677438 |
| 2,2'-Dithiobis(pyridine) |
| di(pyridin-2-yl) disulfide |
| L6X912UPBU |
| Pyridine, 2,2'-dithiodi- |
| MLS001143364 |
| 2-Pyridin-2-yldisulfanylpyridine |
| 2,2 inverted exclamation marka-Dipyridyl disulfide |
| NSC 94055 |
| NSC-94055 |
| NSC677438 |
| NSC 677438 |
| SMR000473199 |
| Pyridine, dithiobis- |
| Di-2-pyridinyl disulfide; Di-2-pyridyl disulfide; NSC 677438; NSC 94055 |
| orthopyridyl disulfide |
| Di(2-Pyridyl) disulfide |
| 2,2'-Dipyridyldisulphide |
| MFCD00006287 |
| aldrithiol |
| 2,2'-disulfanediyldipyridine |
| dithiodipyridine |
| pyridyldisulfide |
| pyridyl disulfide |
| dipyridyldisulfide |
| Aldrithol-2 |
| C10H8N2S2 |
| AldrithiolTM-2 |
| 2-pyridyldisulfide |
| dipyridyl disulfide |
| dipyridyl disulphide |
| 2-pyridyl-disulfide |
| Aldrithiol(TM)-2 |
| di(2-pyridyl)disulfide |
| Pyridine,2'-dithiodi- |
| 2,2'-Diithiodipyridine |
| Pyridine,2'-dithiobis- |
| 2,2'-Dithiodipyridine? |
| 2,2'-dithiobis-pyridine |
| OPSSOrthopyridyl disulfide |
| 2,2'-dipyridyl-disulfide |
| 2.2'-dipyridyl disulfide |
| di(pyridin-2-yl)disulfide |
| UNII-L6X912UPBU |
| 2-,2'-dipyridyl disulfide |
| 2,2'-Dipyridinyl disulfide |
| NCIOpen2_006257 |
| SCHEMBL21174 |
| Aldrithiol(TM)-2, 98% |
| cid_65093 |
| 2,2'-Dithiodipyridine, powder |
| CHEMBL118678 |
| 1,2-Di(pyridin-2-yl)disulfide |
| BDBM60840 |
| HAXFWIACAGNFHA-UHFFFAOYSA- |
| 1,2-bis(pyridin-2-yl)disulfane |
| 1,2-di(pyridine-2-yl)disulfane |
| DTXSID70175517 |
| CHEBI:143170 |
| 2-(2-Pyridinyldisulfanyl)pyridine |
| HMS2805F21 |
| AMY23184 |
| HY-Y1666 |
| NSC94055 |
| EINECS 218-343-1 |
| 2-(2-Pyridinyldisulfanyl)pyridine # |
| AKOS002248740 |
| AC-4591 |
| CS-W008788 |
| 2-(2-(pyridin-2-yl)disulfanyl)pyridine |
| AS-13979 |
| BP-20233 |
| BP-23823 |
| 2,2'-Dithiodipyridine, >=99.0% (GC) |
| D1114 |
| FT-0609299 |
| A26592 |
| EN300-172822 |
| Q-101297 |
| Q4596740 |
| 2-(2-Pyridinyldisulfanyl)pyridine;2,2'-Dipyridinyl disulfide |
| InChI=1/C10H8N2S2/c1-3-7-11-9(5-1)13-14-10-6-2-4-8-12-10/h1-8H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|