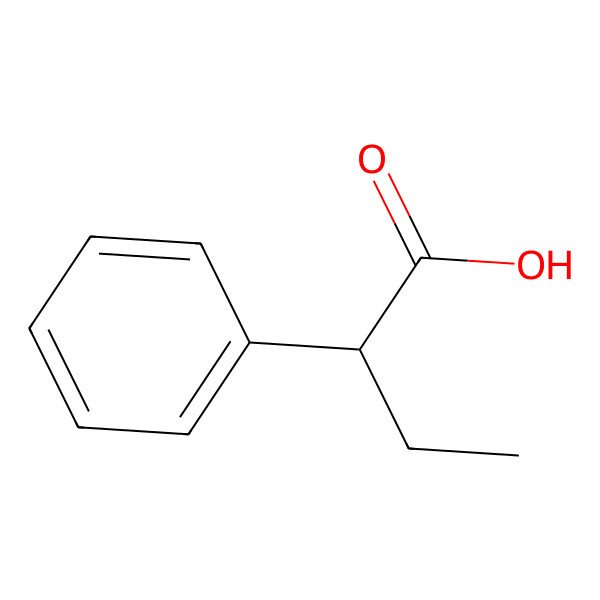
| 2-Phenylbutanoic acid |
| 90-27-7 |
| alpha-Ethylphenylacetic acid |
| Butyric acid, 2-phenyl- |
| Benzeneacetic acid, .alpha.-ethyl- |
| alpha-Phenylbutyric acid |
| Benzeneacetic acid, alpha-ethyl- |
| (+/-)-2-Phenylbutyric acid |
| NSC 1860 |
| 2-phenyl-butyric acid |
| a-Ethylphenylacetic acid |
| MFCD00002667 |
| .alpha.-Phenylbutyric acid |
| (RS)-2-Phenylbutanoic acid |
| alpha-Ethyl-alpha-toluic acid |
| (2RS)-2-Phenylbutanoic Acid |
| CHEBI:86545 |
| NSC1860 |
| S7S079H2C2 |
| NSC-1860 |
| .alpha.-Toluic acid, .alpha.-ethyl- |
| alpha-Phenyl butyric acid |
| 2-Phenylbutanoicacid |
| 2-Phenylburyric acid |
| alpha-Toluic acid, alpha-ethyl- |
| EINECS 201-982-5 |
| BRN 0509876 |
| 2-Ethyl-2-phenylacetic Acid |
| UNII-S7S079H2C2 |
| a-Phenylbutyrate |
| AI3-11228 |
| (2RS)-2-Phenylbutanoic Acid; Primidone Imp. E (EP); Primidone Impurity E |
| 2-Phenylbutyrate |
| 2-phenylbutanoate |
| a-Ethyl-a-toluate |
| a-Ethylphenylacetate |
| alpha-Phenylbutyrate |
| phenyl butanoic acid |
| a-Ethylbenzeneacetate |
| Primidone Impurity E |
| a-Ethyl-a-toluic acid |
| alpha-Ethylphenylacetate |
| Spectrum_001673 |
| alpha-Ethylbenzeneacetate |
| SpecPlus_000897 |
| a-Ethylbenzeneacetic acid |
| (RS)-2-Phenylbutanoate |
| alpha-Ethyl-alpha-toluate |
| Spectrum2_000510 |
| Spectrum3_001664 |
| Spectrum4_000626 |
| Spectrum5_001396 |
| (+)2-phenylbutyric acid |
| bmse000617 |
| A-PHENYLBUTYRIC ACID |
| Cambridge id 5132265 |
| SCHEMBL1715 |
| WLN: QVY2&R |
| .alpha.-Phenyl butyric acid |
| alpha-Ethylbenzeneacetic acid |
| 2-Phenylbutyric acid, 98% |
| BSPBio_003447 |
| KBioGR_001212 |
| KBioSS_002153 |
| 2-09-00-00356 (Beilstein Handbook Reference) |
| DivK1c_006993 |
| .alpha.-Ethylphenylacetic acid |
| SPBio_000439 |
| .alpha.-Phenyl-n-butyric acid |
| (4-CARBOXYPHENYL)ACETONE |
| CHEMBL1616045 |
| KBio1_001937 |
| KBio2_002153 |
| KBio2_004721 |
| KBio2_007289 |
| KBio3_002667 |
| DTXSID90861682 |
| (.+/-.)-2-Phenylbutyric acid |
| (.+/-.)-2-Phenylbutanoic acid |
| AMY40956 |
| s6086 |
| AKOS000120324 |
| AKOS016040111 |
| (+/-)-2-PHENYLBUTANOIC ACID |
| BS-3887 |
| CS-W017910 |
| HY-W017194 |
| SB44765 |
| AC-10409 |
| PD065554 |
| SY036812 |
| PRIMIDONE IMPURITY E [EP IMPURITY] |
| FT-0605052 |
| FT-0605258 |
| FT-0613342 |
| P0164 |
| EN300-20606 |
| E78161 |
| A843486 |
| J-520787 |
| W-100334 |
| Q27159231 |
| F2191-0104 |
| Z104479150 |
| (+/-)-2-Phenylbutyric acid, Vetec(TM) reagent grade, 98% |
| ?-Ethylphenylacetic acid; (+/-)-2-Phenylbutyric acid; (RS)-2-Phenylbutanoic acid; 2-Ethyl-2-phenylacetic Acid |
| InChI=1/C10H12O2/c1-2-9(10(11)12)8-6-4-3-5-7-8/h3-7,9H,2H2,1H3,(H,11,12 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|