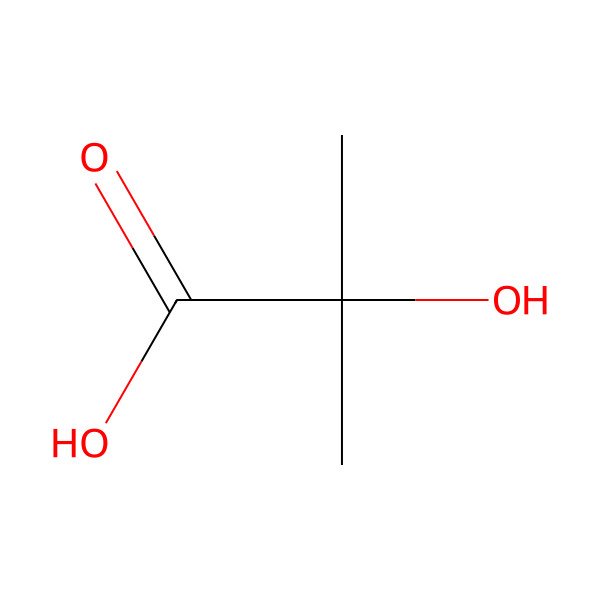
| 594-61-6 |
| 2-Methyllactic acid |
| 2-Hydroxy-2-methylpropanoic acid |
| Acetonic acid |
| alpha-Hydroxyisobutyric acid |
| 2-Hydroxy-2-methylpropionic acid |
| Hydroxydimethylacetic acid |
| Lactic acid, 2-methyl- |
| Propanoic acid, 2-hydroxy-2-methyl- |
| alpha-Hydroxyisobutanoic acid |
| a-hydroxyisobutyric acid |
| 2-hydroxy-2-methyl-propanoic acid |
| MFCD00004459 |
| .alpha.-Hydroxyisobutyric acid |
| alpha-Hydroxy-alpha-methylpropanoic acid |
| HIBA |
| .alpha.-Hydroxyisobutanoic acid |
| 2-hydroxy-2-methyl-propionic acid |
| NSC 4505 |
| (CH3)2COHCOOH |
| NSC 402158 |
| DMW250U2HF |
| DTXSID4032954 |
| CHEBI:50129 |
| NSC-4505 |
| 2-Methyllactate |
| NSC-402158 |
| 2-Hydroxyisobutyricacid |
| .alpha.-Hydroxy-.alpha.-methylpropanoic acid |
| alpha-Hydroxyisobutyrate |
| alpha-hydroxyisobutanoate |
| 2-HYDROXY-2-METHYL-D3-PROPIONIC-3,3,3-D3 ACID |
| Acetonate |
| alpha-Hydroxy-alpha-methylpropanoate |
| UNII-DMW250U2HF |
| Lactic acid, 2-methyl-, L- |
| 2-hydroxy-2-methylpropanoicacid |
| Hydroxyisobutyrate |
| alpha-HIB |
| l-2-methyllactate |
| a-Hydroxyisobutyrate |
| EINECS 209-848-8 |
| a-Hydroxyisobutanoate |
| Hydroxydimethylacetate |
| hydroxyisobutyric acid |
| a-HIB |
| l-2-methyllactic acid |
| a-Hydroxyisobutanoic acid |
| AI3-31313 |
| 2-hydroxy isobutyric acid |
| 2-hydroxy-isobutyric acid |
| METHYL LACTIC ACID |
| 2-hydroxy-iso-butyric acid |
| a-Hydroxy-a-methylpropanoate |
| alpha -hydroxyisobutyric acid |
| alpha-hydroxy isobutyric acid |
| alpha-hydroxy-isobutyric acid |
| SCHEMBL29373 |
| 2-Hydroxy-2-methylpropionate |
| 2-Hydroxy-2-methylpropionsaeure |
| 2-Methyl-2-hydroxypropionsaeure |
| a-Hydroxy-a-methylpropanoic acid |
| CHEMBL1345148 |
| DTXCID2012954 |
| 2-HYDROXYISOBUTANOIC ACID |
| 2-methyl-2-hydroxypropanoic acid |
| NSC4505 |
| acido 2-hidroxi-2-metilpropionico |
| METHYL LACTIC ACID [INCI] |
| 2-hydroxy-2-methyl propionic acid |
| alpha-Hydroxyisobutyric acid, 98% |
| alpha-Hydroxyisobutyric acid, 99% |
| acide 2-hydroxy-2-methylpropanoique |
| STR04462 |
| Tox21_200123 |
| LMFA01050417 |
| NSC402158 |
| s6307 |
| Propanoic acid, 2-methyl-2-hydroxy- |
| AKOS001310472 |
| CS-W016640 |
| HY-W015924 |
| JC10155 |
| alpha-hydroxy-alpha-methylpropionic acid |
| s12287 |
| NCGC00090926-01 |
| NCGC00090926-02 |
| NCGC00257677-01 |
| CAS-594-61-6 |
| PD123991 |
| SY001526 |
| ISOBUTYRIC ACID, .ALPHA.-HYDROXY- |
| A8360 |
| FT-0612616 |
| FT-0669843 |
| M0360 |
| EN300-59285 |
| C21297 |
| H-6400 |
| AB00984267-01 |
| Q2735617 |
| alpha-Hydroxyisobutyric acid, purum, >=98.0% (HPLC) |
| Z220542620 |
| InChI=1/C4H8O3/c1-4(2,7)3(5)6/h7H,1-2H3,(H,5,6 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|