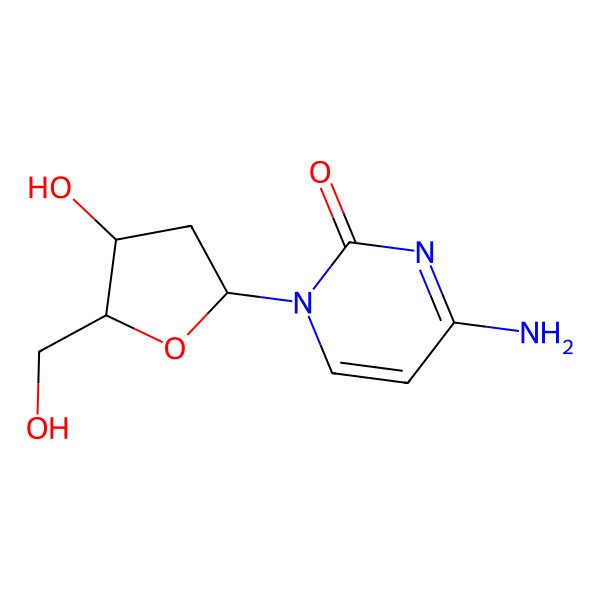
| deoxycytidine |
| 951-77-9 |
| CYTIDINE, 2'-DEOXY- |
| Cytosine deoxyriboside |
| Deoxyribose cytidine |
| dCYD |
| 4-Amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one |
| 207121-53-7 |
| Desoxycytidin [German] |
| Cytosine, deoxyribonucleoside |
| Desoxycytidin |
| Doxecitine |
| d-cytidine |
| 1beta-2'-Deoxyribofuranosylcytosine, d- |
| 2'-Deoxycytidine monohydrate |
| EINECS 213-454-1 |
| BRN 0087567 |
| 1-(2-Deoxy-beta-D-ribofuranosyl)cytosine |
| 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one |
| DC |
| CHEBI:15698 |
| UNII-0W860991D6 |
| Cytidine, 2'-deoxy-, labeled with tritium |
| 0W860991D6 |
| Desoxycytidine |
| 4-25-00-03662 (Beilstein Handbook Reference) |
| Deoxycytidine;Cytosine deoxyriboside;Deoxyribose cytidine |
| 4-Amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)-2(1H)-pyrimidinone |
| MFCD00006547 |
| 2(1H)-Pyrimidinone, 4-amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)- |
| 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydropyrimidin-2-one |
| 2'-deoxy-cytidine |
| 56905-41-0 |
| DCZ |
| deoxy-Cytidine |
| 2-Deoxycytidine |
| 2' Deoxycytidine |
| 2-deoxy-Cytidine |
| 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one |
| DOXECITINE [INN] |
| DOXECITINE [USAN] |
| DEOXYCYTIDINE [INCI] |
| SCHEMBL23178 |
| 2'-dC |
| CHEMBL66115 |
| DTXSID70883620 |
| CKTSBUTUHBMZGZ-SHYZEUOFSA-N |
| 4-amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)pyrimidin-2(1H)-one |
| HY-D0184 |
| BDBM50367094 |
| HG1097 |
| 2'-Deoxycytidine, >=99% (HPLC) |
| AKOS015896791 |
| AC-8210 |
| AM83951 |
| DB02594 |
| 1-(2-Deoxy-b-D-ribofuranosyl)cytosine |
| 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one |
| NCGC00142493-01 |
| AS-14313 |
| BP-58643 |
| LS-59066 |
| 1-(2-deoxy-.beta.-d-ribofuranosyl)cytosine |
| CS-0010105 |
| D3583 |
| MT-1621 COMPONENT 2'-DEOXYCYTIDINE |
| 1-(2-Deoxy-beta-delta-ribofuranosyl)cytosine |
| C00881 |
| EN300-6477283 |
| A845205 |
| Q422504 |
| 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-Cytosine |
| J-700038 |
| BRD-K91822704-001-01-9 |
| 1-(2-deoxy-beta-delta-erythro-pentofuranosyl)-Cytosine |
| Z3072884208 |
| 4-Amino-1-(2-deoxy-b-D-erythro-pentofuranosyl)-2(1H)-pyrimidinone |
| 4-amino-1-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-2(1h)-pyrimidinone |
| 4-Amino-1-(2-deoxy-beta-delta-erythro-pentofuranosyl)-2(1H)-pyrimidinone |
|
There are more than 10 synonyms. If you wish to see them all click here.
|