| 18531-94-7 |
| 602-09-5 |
| 18531-99-2 |
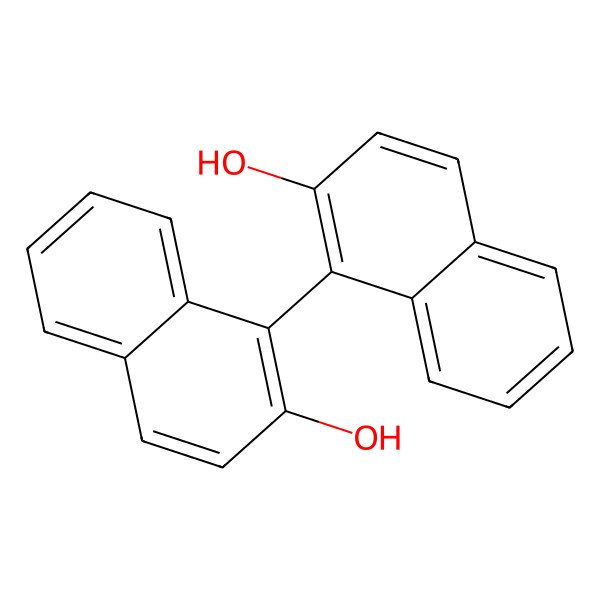
| (S)-(-)-1,1'-Bi-2-naphthol |
| (R)-(+)-1,1'-Bi-2-naphthol |
| [1,1'-Binaphthalene]-2,2'-diol |
| binol |
| (S)-BINOL |
| 1,1'-Binaphthyl-2,2'-diol |
| beta-Binaphthol |
| (R)-BINOL |
| 1-(2-hydroxynaphthalen-1-yl)naphthalen-2-ol |
| (S)-[1,1'-binaphthalene]-2,2'-diol |
| (R)-[1,1'-Binaphthalene]-2,2'-diol |
| (R)-Binaphthol |
| (S)-Binaphthol |
| r-(+)-1,1'-bi-2-naphthol |
| 2,2'-Dihydroxy-1,1'-binaphthyl |
| 2,2'-Dinaphthol |
| (R)-1,1'-Bi-2-naphthol |
| (R)-(+)-1,1'-Bi(2-naphthol) |
| Chiral binaphthol |
| Bis-beta-naphthol |
| (R)-(+)-BINOL |
| (S)-(-)-BINOL |
| 1,1'-Bis-2-naphthol |
| 2,2'-Dihydroxydinaphthyl |
| Binol, (+)- |
| 2,2'-Dihydroxy-1,1'-dinaphthyl |
| 2,2'-Dihydroxybinaphthalene |
| MFCD00004068 |
| (R)-Bi-2-naphthol |
| (-)-Binol |
| (+)-Bi-2-naphthol |
| (S)-(-)-1,1'-Bi(2-naphthol) |
| (+)-1,1'-Bis(2-naphthol) |
| (-)-1,1'-Bis(2-naphthol) |
| Binol, (-)- |
| (r)-(+)-1,1-bi-2-naphthol |
| (+)-2,2'-Binaphthol |
| (-)-2,2'-Binaphthol |
| .beta.-Binaphthol |
| 2,2'-Dihydroxy-1,1'-binaphthalene |
| alpha-Binaphthyl-2,2'-diol |
| (s)-1,1'-bi-2-naphthol |
| (S)-2,2'-Dihydroxy-1,1'-binaphthyl |
| Bis-.beta.-naphthol |
| (+)-2,2'-Dihydroxy-1,1'-dinaphthyl |
| (+)-1,1'-Bi-2-naphthol |
| (-)-1,1'-Bi-2-naphthol |
| (S)-2,2'-Dihydroxy-1,1'-binaphthalene |
| Binol (R)-(+)-form [MI] |
| Binol (S)-(-)-form [MI] |
| (1S)-[1,1'-binaphthalene]-2,2'-diol |
| (-)-Bi-beta-naphthol |
| (+)-Bi-beta-Naphthol |
| (S)-(-)-2,2'-Dihydroxy-1,1'-binaphthyl |
| EINECS 210-014-0 |
| NSC 27049 |
| (S)-(-)-2,2'-Dihydroxy-1,1'-binaphthalene |
| UNII-54OT5RRV4C |
| UNII-M6IDZ128WT |
| BRN 0997518 |
| 54OT5RRV4C |
| M6IDZ128WT |
| (+)-2,2'-dihydroxy-1,1'-binaphthyl |
| (R)-(1,1'-Binaphthalene)-2,2'-diol |
| (+/-)-1,1'-Binaphthalene-2,2'-diol |
| (+)-2,2'-Dihydroxy-1,1'-binaphthalene |
| AI3-19388 |
| (1,1'-BINAPHTHALENE)-2,2'-DIOL |
| .alpha.-Binaphthyl-2,2'-diol |
| (+)-(R)-2,2'-dihydroxy-1,1'-binaphthyl |
| (+)-(1R)-(1,1'-Binaphthalene)-2,2'-diol |
| (-)-(1S)-(1,1'-Binaphthalene)-2,2'-diol |
| UNII-25AB254328 |
| (1,1'-Binaphthalene)-2,2'-diol, (R)-(+)- |
| (1,1'-Binaphthalene)-2,2'-diol, (S)-(-)- |
| [1,1']Binaphthalenyl-2,2'-diol |
| (S)-(-)-1,1-Bi-2-naphthol |
| (RS)-1,1'-Bi-2,2'-naphthol |
| NSC-27049 |
| (S)-1,1'-binaphthalene-2,2'-diol |
| (r)-2,2'-dihydroxy-[1,1']-binaphthyl |
| (+/-)-2,2'-Dihydroxy-1,1'-dinaphthyl |
| 25AB254328 |
| (R)-(+)-2,2'-Dihydroxy-1,1'-binaphthyl |
| [1,1'-Binaphthalene]-2,2'-diol, (1S)- |
| EC 606-050-5 |
| (R)-1-(2-Hydroxynaphthalen-1-yl)naphthalen-2-ol |
| 4-06-00-07020 (Beilstein Handbook Reference) |
| s-(-)-1,1'-bi-2-naphthol |
| Benzamide,N-(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)-2-[2-(dimethylamino)ethoxy]- |
| 1-(2-hydroxy-1-naphthalenyl)-2-naphthalenol |
| 1-(2-oxidanylnaphthalen-1-yl)naphthalen-2-ol |
| (+/-)-1,1'-bi-2-naphthol |
| 2,1'-binaphthyl |
| (r,s)-binol |
| (+/-)-1,1'-Bi(2-naphthol) |
| 2,1'-binaphthalene |
| (+)-beta-Dinaphthol |
| (1r)-[1,1'-binaphthalene]-2,2'-diol |
| s-(-)-2,2'-dihydroxy-1,1'-dinaphthyl |
| (s)-2,2'-binaphthol |
| BINOL [MI] |
| (R)-2,2'-Binaphthol |
| 1,1''-Bi-2-naphthol |
| (r)-(+)-bi-2-naphthol |
| SCHEMBL29027 |
| 1,1a(2)-Bi-2-naphthol |
| BINOL, (R)- |
| BINOL, (S)- |
| CHEMBL138718 |
| (5)-1,1'-bi-2-naphthol |
| DTXSID9060526 |
| (+/-)-BINOL |
| (aS)-1,1'-Bi(2-naphthol) |
| CHEBI:189881 |
| (+)-BI-.BETA.-NAPHTHOL |
| (-)-BI-.BETA.-NAPHTHOL |
| 1,1'-Bi-2-naphthol, 99% |
| 2,2'-dihydroxyl-1,1'-binaphthyl |
| BCP23136 |
| BCP23230 |
| BCP30910 |
| CS-D1439 |
| NSC27049 |
| (r)-1,1'-binaphthyl-2,2'-diol |
| (s)-1,1'-binaphthyl-2,2'-diol |
| [1,1']-binaphthalenyl-2,2'-diol |
| (+/-)-BI-.BETA.-NAPHTHOL |
| (S)-(-)-1,1'-bi-2-naphtol |
| (+)-1,1'-Binaphthyl-2,2'-diol |
| (-)-1,1'-Binaphthyl-2,2'-diol |
| (R)-(+)-1,1`-Bi-2-naphthol |
| (RS)-1,1'-BI-2-NAPHTHOL |
| (S)-(+)-1,1`-Bi-2-naphthol |
| (S)-(-)-1,1`-Bi-2-naphthol |
| AKOS000280003 |
| AKOS015950645 |
| AKOS015950732 |
| rac-2,2'-dihydroxy-1,1'-binaphthyl |
| (R)-1,1'-Binaphthalene-2,2'-diol |
| (S)-(-)-1,1\'-bi-2-naphthol |
| AC-2795 |
| AC-6133 |
| AM62789 |
| CS-W018498 |
| GS-3437 |
| (pR)-1,1'-Binaphthalene-2,2'-diol |
| (pS)-1,1'-Binaphthalene-2,2'-diol |
| (R)-(+)-1,1\'-Bi-2-naphthol |
| rac-[1,1'-Binaphthalene]-2,2'-diol |
| BP-21310 |
| LS-43999 |
| LS-44000 |
| (r)-2,2'-dihydroxy-1,1'-binaphthalene |
| r-(+)-2,2'-dihydroxy-1,1'-dinaphthyl |
| (+/-)-1,1'-BIS(2-NAPHTHOL) |
| B0461 |
| B1100 |
| B1142 |
| FT-0602420 |
| FT-0604444 |
| FT-0605200 |
| [1,1'-Binaphthalene]-2,2'-diol, (1R)- |
| 2,2'-DIHYDROXY-[1,1']-BINAPHTHYL |
| EN300-67379 |
| (R)-(+)-1,1'-Bi(2-naphthol), 99% |
| (S)-(-)-1,1'-Bi(2-naphthol), 99% |
| (+/-)-1,1'-BINAPHTHYL-2,2'-DIOL |
| (R)-(+)-2,2'-Dihydroxy-1,1'-binaphthalene |
| RACEMIC 2,2'-DIHYDROXY-1,1'-BINAPHTHYL |
| A812947 |
| A832643 |
| Q161292 |
| (+/-)-2,2'-DIHYDROXY-1,1'-BINAPHTHYL |
| J-011889 |
| J-503719 |
| Q-103560 |
| Q-103561 |
| Q-200048 |
| (+/-)-2,2'-DIHYDROXY-1,1'-BINAPHTHALENE |
| (R)-(+)-1 |
| F0001-0669 |
| (+/-)-1,1'-Bi(2-naphthol), puriss., >=99.0% (NT) |
| (R)-(+)-1,1'-Bi(2-naphthol), puriss., >=99.0% (sum of enantiomers, HPLC) |
| InChI=1/C20H14O2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12,21-22 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|