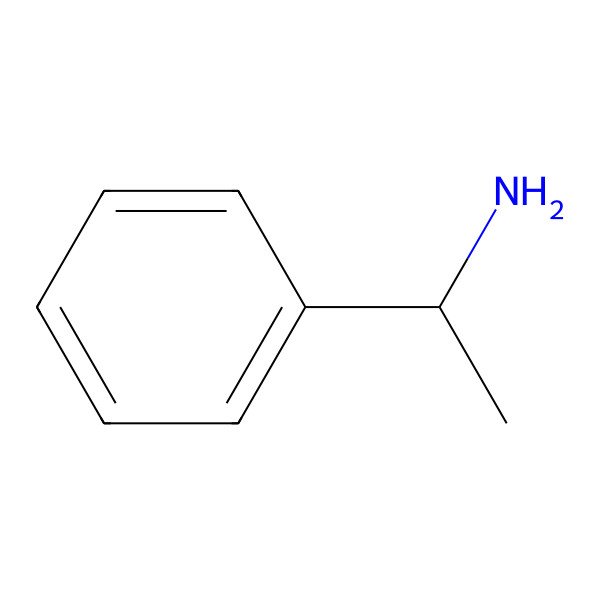
| 1-Phenylethylamine |
| 618-36-0 |
| ALPHA-METHYLBENZYLAMINE |
| DL-alpha-Methylbenzylamine |
| alpha-Phenylethylamine |
| 1-Phenethylamine |
| 98-84-0 |
| alpha-Aminoethylbenzene |
| 1-Amino-1-phenylethane |
| 1-phenylethan-1-amine |
| a-methylbenzylamine |
| alpha-Methylbenzenemethanamine |
| alpha-Phenethylamine |
| DL-1-Phenylethylamine |
| Sumine 2079 |
| 1-Fenylethylamin |
| Ethanamine, 1-phenyl- |
| Ethylamine, 1-phenyl- |
| a-phenylethylamine |
| 1-Phenyl-ethylamine |
| Benzenemethanamine, .alpha.-methyl- |
| (1-Aminoethyl)benzene |
| DL-a-methylbenzyla |
| Benzene, (1-amino-ethyl)- |
| Benzylamine, .alpha.-methyl- |
| .alpha.-Phenethylamine |
| .alpha.-Phenylethylamine |
| a-methylbenzenemethanamine |
| .alpha.-Methylbenzylamine |
| HZ9DM6B2MT |
| CHEBI:670 |
| CHEMBL278059 |
| (+/-)-alpha-Methylbenzylamine |
| NSC-8391 |
| Benzenemethaneamine, .alpha.-methyl- |
| (S)-.alpha.-Methylbenzenemethanamine |
| MFCD00008069 |
| Benzenemethanamine, .alpha.-methyl-, (S)- |
| (S)-alpha-methyl benzylamine |
| UNII-HZ9DM6B2MT |
| 1-Fenylethylamin [Czech] |
| Benzylamine, alpha-methyl- |
| (S)-(-)-a-methyl-benzylamine |
| a-phenethylamine |
| Timepidiumbromide |
| (S)-(-)-.alpha.-Methylbenzylamine |
| HSDB 2742 |
| l-phenylethylamine |
| Benzenemethanamine, alpha-methyl- |
| a-aminoethylbenzene |
| Benzenemethaneamine, alpha-methyl- |
| NSC 8391 |
| 1-phenylethyl amine |
| EINECS 202-706-6 |
| EINECS 210-545-8 |
| phenylethan-1-amine |
| 1-Phenyl ethylamine |
| DL-1-phenethylamine |
| alpha-methylbezylamine |
| (1-phenylethyl)amine |
| 1-phenyl-ethyl-amine |
| 1-Phenylethanamine # |
| rac-1-phenylethanamine |
| dl-1-phenylethyl amine |
| 1-Phenyl-1-ethanamine |
| alpha-methyl benzylamine |
| alpha-methyl-benzylamine |
| alpha-methylbenzyl amine |
| (rs)-1-phenylethylamine |
| 1(r,s)-phenylethylamine |
| AI3-03116 |
| (+)-1-phenylethylamine |
| (-)-1-phenylethylamine |
| alpha-methyl benzyl amine |
| DL-alpha-Phenylethylamine |
| (-)alpha-phenylethylamine |
| racemic 1-phenylethanamine |
| racemic 1-phenylethylamine |
| SCHEMBL830 |
| (-)-alpha-phenylethylamine |
| EC 210-545-8 |
| (+) alpha-methylbenzylamine |
| (+)-alpha-methylbenzylamine |
| (+)1-phenylethan-1-amine |
| (+/-)-1-phenylethanamine |
| (-)-alpha-methylbenzylamine |
| (+/-)-1-Phenylethylamine |
| (RS)-alpha-methylbenzylamine |
| (-)-.alpha.-Phenethylamine |
| (+)-alpha-methyl-benzylamine |
| (-) alpha-methyl-benzylamine |
| (-)-alpha-methyl-benzylamine |
| WLN: 1M1R |
| (+/-)-1-phenyl-ethanamine |
| (+/-)-1-Phenyl-ethylamine |
| alpha-Methylbenzylamine, 99% |
| racemic alpha-methylbenzylamine |
| (+)-alpha-methyl benzyl amine |
| (+/-)-1-(phenyl)ethylamine |
| (-) alpha methyl benzyl amine |
| SCHEMBL4701869 |
| 1-PHENETHYLAMINE [HSDB] |
| .ALPHA.-AMINOETHYLBENZENE |
| L(-)-.alpha.-Methylbenzylamine |
| DTXSID40862301 |
| L-(-)-.alpha.-Phenylethylamine |
| NSC8391 |
| (+/-)- alpha -Methylbenzylamine |
| (-)-alpha-methylbenzenemethanamine |
| BCP32849 |
| DL-.ALPHA.-METHYLBENZYLAMINE |
| (R)-.alpha.-Methylbenzenemethanamine |
| BDBM50023171 |
| STK397443 |
| AKOS000119070 |
| AKOS016039387 |
| racemic alpha-methyl-benzenemethanamine |
| (RS)-.ALPHA.-METHYLBENZYLAMINE |
| .ALPHA.-METHYLBENZENEMETHANAMINE |
| .ALPHA.-METHYLBENZYLAMINE [MI] |
| CS-W013564 |
| PS-4601 |
| (+/-)-.ALPHA.-PHENETHYLAMINE |
| (+/-)-.ALPHA.-PHENYLETHYLAMINE |
| (R,S)-.ALPHA. METHYLBENZYLAMINE |
| AS-80972 |
| Benzenemethanamine, .alpha.-methyl-, (R)- |
| BENZYLAMINE, .ALPHA.-METHYL-, DL- |
| AM20060838 |
| Benzylamine, .alpha.-methyl-, (.+/-.)- |
| FT-0601072 |
| FT-0604486 |
| FT-0658781 |
| M0165 |
| EN300-17925 |
| C02455 |
| D77753 |
| DL-|A-Methylbenzylamine DL-|A-Phenylethylamine |
| (S)-1-phenylethanamine;(1S)-1-phenylethanamine |
| Benzenemethanamine, .alpha.-methyl-, (.+/-.)- |
| N-FMOC-AMINO-4-KETOCYCLOHEXYLCARBOXYLICACID |
| Q3560549 |
| W-105090 |
| Z57102377 |
| F0798-0597 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|