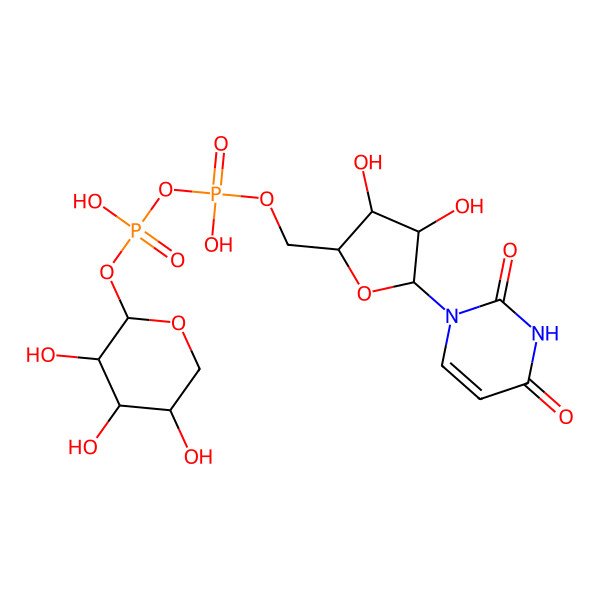
UDP-xylose
| Internal ID | 924a1dd2-8ebc-429a-8b75-52a195ff0040 |
| Taxonomy | Nucleosides, nucleotides, and analogues > Pyrimidine nucleotides > Pyrimidine ribonucleotides > Pyrimidine ribonucleoside diphosphates |
| IUPAC Name | [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl] hydrogen phosphate |
| SMILES (Canonical) | C1C(C(C(C(O1)OP(=O)(O)OP(=O)(O)OCC2C(C(C(O2)N3C=CC(=O)NC3=O)O)O)O)O)O |
| SMILES (Isomeric) | C1[C@H]([C@@H]([C@H]([C@H](O1)OP(=O)(O)OP(=O)(O)OC[C@@H]2[C@H]([C@H]([C@@H](O2)N3C=CC(=O)NC3=O)O)O)O)O)O |
| InChI | InChI=1S/C14H22N2O16P2/c17-5-3-28-13(11(22)8(5)19)31-34(26,27)32-33(24,25)29-4-6-9(20)10(21)12(30-6)16-2-1-7(18)15-14(16)23/h1-2,5-6,8-13,17,19-22H,3-4H2,(H,24,25)(H,26,27)(H,15,18,23)/t5-,6-,8+,9-,10-,11-,12-,13-/m1/s1 |
| InChI Key | DQQDLYVHOTZLOR-OCIMBMBZSA-N |
| Popularity | 135 references in papers |
| Molecular Formula | C14H22N2O16P2 |
| Molecular Weight | 536.28 g/mol |
| Exact Mass | 536.04445661 g/mol |
| Topological Polar Surface Area (TPSA) | 271.00 Ų |
| XlogP | -6.60 |
| UDP-xylose |
| UDP-alpha-D-xylose |
| URIDINE DIPHOSPHATE XYLOSE |
| URIDINE-5'-DIPHOSPHATE-XYLOPYRANOSE |
| 3616-06-6 |
| UDP-D-xylose |
| Uridine 5'-(trihydrogen diphosphate), P'-alpha-D-xylopyranosyl ester |
| UDP-ALPHA-D-XYLOPYRANOSE |
| uridine 5'-[3-(alpha-D-xylopyranosyl) dihydrogen diphosphate] |
| UDX |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 97.56% | 96.09% |
| CHEMBL226 | P30542 | Adenosine A1 receptor | 95.44% | 95.93% |
| CHEMBL1075162 | Q13304 | Uracil nucleotide/cysteinyl leukotriene receptor | 94.52% | 80.33% |
| CHEMBL2123 | P51582 | Pyrimidinergic receptor P2Y4 | 92.33% | 93.39% |
| CHEMBL2581 | P07339 | Cathepsin D | 91.74% | 98.95% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 88.97% | 91.11% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 88.91% | 97.09% |
| CHEMBL5957 | P21589 | 5'-nucleotidase | 88.58% | 97.78% |
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 87.99% | 97.25% |
| CHEMBL4016 | P42262 | Glutamate receptor ionotropic, AMPA 2 | 87.89% | 86.92% |
| CHEMBL3401 | O75469 | Pregnane X receptor | 83.07% | 94.73% |
| CHEMBL3230 | O95977 | Sphingosine 1-phosphate receptor Edg-6 | 82.63% | 94.01% |
| CHEMBL4040 | P28482 | MAP kinase ERK2 | 82.14% | 83.82% |
| CHEMBL225 | P28335 | Serotonin 2c (5-HT2c) receptor | 81.84% | 89.62% |
| CHEMBL2635 | P51452 | Dual specificity protein phosphatase 3 | 81.77% | 94.00% |
| CHEMBL3137261 | O14744 | PRMT5/MEP50 complex | 81.03% | 100.00% |
| CHEMBL1806 | P11388 | DNA topoisomerase II alpha | 80.96% | 89.00% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 80.44% | 95.89% |
| CHEMBL255 | P29275 | Adenosine A2b receptor | 80.23% | 98.59% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 80.19% | 94.45% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| Glycine max |
| PubChem | 19235 |
| LOTUS | LTS0137624 |
| wikiData | Q27089069 |