| 6138-23-4 |
| Trehalose dihydrate |
| D-Trehalose dihydrate |
| alpha,alpha-Trehalose dihydrate |
| a,a-Trehalose |
| TREHALOSE, DIHYDRATE |
| UNII-7YIN7J07X4 |
| 7YIN7J07X4 |
| DTXSID3047972 |
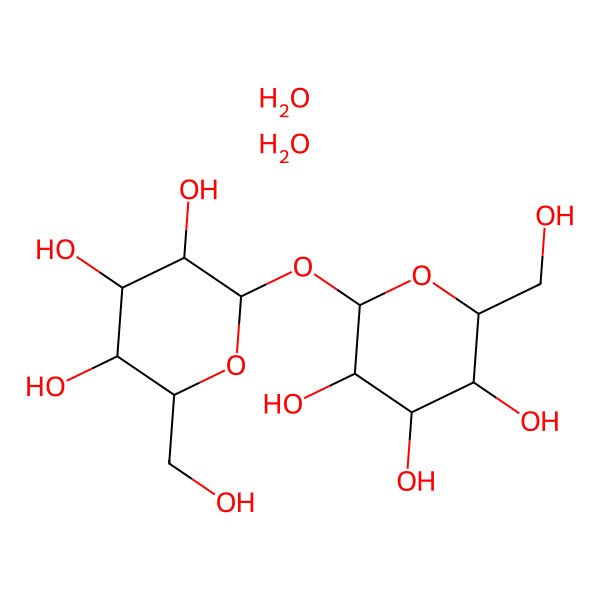
| (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-3,4,5-triol;dihydrate |
| MFCD00071594 |
| alpha-D-Glucopyranoside, alpha-D-glucopyranosyl, dihydrate |
| (2R,2'R,3S,3'S,4S,4'S,5R,5'R,6R,6'R)-6,6'-Oxybis(2-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol) dihydrate |
| alp,alp.-Trehalose |
| MYCOSE DIHYDRATE |
| a,a-Trehalose dihydrate |
| dextro-trehalose dihydrate |
| D-Trehalose; ,-Trehalose |
| SCHEMBL166078 |
| CHEMBL3188936 |
| DTXCID9027948 |
| FEMA NO. 4600 |
| TREHALOSE DIHYDRATE [MI] |
| Trehalose dihydrate, cell culture |
| DPVHGFAJLZWDOC-PVXXTIHASA-N |
| HMS3885G16 |
| Tox21_201106 |
| Trehalose (for injection) (sterile) |
| TREHALOSE DIHYDRATE [WHO-DD] |
| a-d-glucopyranosyl-a-d-glucopyranoside |
| AKOS016010526 |
| CCG-268385 |
| C12-H22-O11.2H2-O |
| NCGC00258658-01 |
| AS-11785 |
| BP-20564 |
| TREHALOSE DIHYDRATE [EP MONOGRAPH] |
| CAS-6138-23-4 |
| D-(+)-Trehalose dihydrate, p.a., 99% |
| 2,2'-(1,6-Hexanediyldioxy)bisbenzaldehyde |
| alp.-D-Glucopyranosyl-alp.-D-glucopyranoside |
| D-Trehalose dihydrate; ,-Trehalose dihydrate |
| S3992 |
| .ALPHA., .ALPHA.-TREHALOSE DIHYDRATE |
| D70602 |
| A833195 |
| D-Trehalose dihydrate 1000 microg/mL in Acetonitrile |
| Q27269028 |
| D-(+)-Trehalose dihydrate, for microbiology, >=99.0% |
| D-(+)-Trehalose dihydrate, SAJ special grade, >=99.0% |
| .alpha.-D-Glucopyranoside, .alpha.-D-glucopyranosyl, dihydrate |
| D-(+)-Trehalose dihydrate, Vetec(TM) reagent grade, >=99% |
| Trehalose, United States Pharmacopeia (USP) Reference Standard |
| .ALPHA.-D-GLUCOPYRANOSYL-.ALPHA.-D-GLUCOPYRANOSIDE DIHYDRATE |
| D-(+)-Trehalose dihydrate, from Saccharomyces cerevisiae, >=99% |
| Trehalose dihydrate, European Pharmacopoeia (EP) Reference Standard |
| D-(+)-Trehalose dihydrate, from Saccharomyces cerevisiae, >=98.5% (HPLC) |
| Trehalose, Pharmaceutical Secondary Standard; Certified Reference Material |
| D-(+)-Trehalose dihydrate (D-Trehalose dihydrate; alpha,alpha-Trehalose dihydrate) |
| (2R,2'R,3S,3'S,4S,4'S,5R,5'R,6R,6'R)-6,6'-Oxybis(2-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol)dihydrate |
| 2-(hydroxymethyl)-6-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxane-3,4,5-triol dihydrate |
| 2-(hydroxymethyl)-6-[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-tetrahydropyran-3,4,5-triol dihydrate;D-(+)-Trehalose dihydrate |
| D-(+)-Trehalose dihydrate, from Saccharomyces cerevisiae, BioReagent, plant cell culture tested |
| D-(+)-Trehalose dihydrate, from Saccharomyces cerevisiae, powder, BioReagent, suitable for cell culture, suitable for insect cell culture, >=99% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|