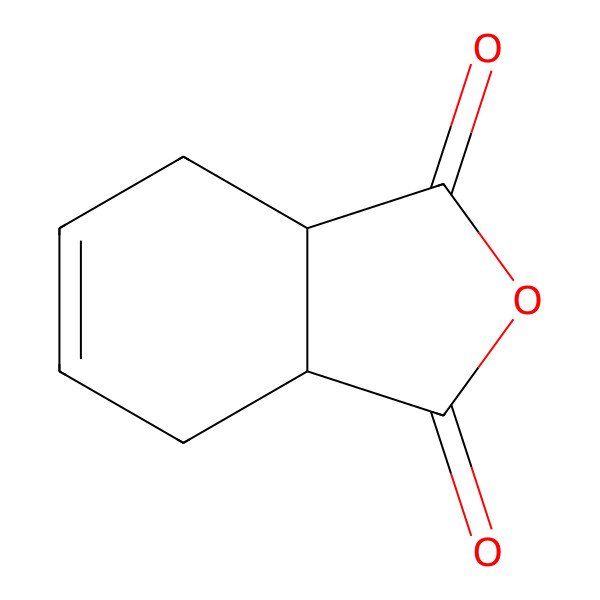
| TETRAHYDROPHTHALIC ANHYDRIDE |
| 1,2,3,6-Tetrahydrophthalic anhydride |
| 3a,4,7,7a-Tetrahydroisobenzofuran-1,3-dione |
| THPA |
| Tetrahydroftalanhydrid |
| Rikacid TH |
| Tetrahydrophthalic acid anhydride |
| 1,3-Isobenzofurandione, 3a,4,7,7a-tetrahydro- |
| Memtetrahydro phtalic anhydride |
| 3a,4,7,7a-Tetrahydro-2-benzofuran-1,3-dione |
| 4-Cyclohexene-1,2-dicarboxylic acid anhydride |
| 4-Cyclohexene-1,2-dicarboxylic anhydride |
| Anhydrid kyseliny tetrahydroftalove |
| 1,2,3,6-Tetrahydrophthalic acid anhydride |
| 1,3-Isobenzofurandione, tetrahydro- |
| NSC 82642 |
| Tetrahydroftalanhydrid [Czech] |
| HSDB 846 |
| Phthalic anhydride, 1,2,3,6-tetrahydro- |
| EINECS 201-605-4 |
| EINECS 247-570-9 |
| delta(4)-Tetrahydrophthalic anhydride |
| BRN 0082340 |
| delta(sup 4)-Tetrahydrophthalic anhydride |
| delta-(sup4)-Tetrahydrophthalic anhydride |
| AI3-22626 |
| Anhydrid kyseliny tetrahydroftalove [Czech] |
| DTXSID3026516 |
| 1,3,3a,4,7,7a-hexahydro-2-benzofuran-1,3-dione |
| MFCD00065335 |
| EC 201-605-4 |
| 26266-63-7 |
| 5-17-11-00134 (Beilstein Handbook Reference) |
| Tetrahydrophthalic Anhydride(THPA);3a,4,7,7a-Tetrahydroisobenzofuran-1,3-dione;3a,4,7,7a-Tetrahydroisobenzofuran-1,3-dione |
| NSC-82642 |
| Rikacid THPA |
| Epiclon B 570H |
| 4,7,3a,7a-tetrahydroisobenzofuran-1,3-dione |
| 4-Cyclohexene-1, trans- |
| CHEMBL8001 |
| NCIOpen2_001003 |
| Tetrahydrophthalsaeureanhydrid |
| UN 2698 (Salt/Mix) |
| SCHEMBL169100 |
| WLN: T56 BVOV GUTJ |
| 3a,4,7,7a-Tetrahydro-isobenzofuran-1,3-dione |
| DTXCID306516 |
| 1, 3a,4,7,7a-tetrahydro- |
| CHEBI:180650 |
| 1,3,6-Tetrahydrophthalic anhydride |
| KBH 1098 |
| NSC82642 |
| Delta 4-Tetrahydrophthalic anhydride |
| Tox21_202210 |
| B 570H |
| BBL011743 |
| HK 019 |
| NSC292900 |
| STL163382 |
| .DELTA.4-Tetrahydrophthalic anhydride |
| 1 2 3 6-Tetrahydrophthalic anhydride |
| AKOS000119224 |
| Phthalic anhydride,2,3,6-tetrahydro- |
| .delta.-4-Tetrahydrophthalic anhydride |
| 1236-Tetrahydrophthalic acid anhydride |
| CS-W019332 |
| Cyclohexene-4 5-dicarboxylic anhydride |
| NSC-292900 |
| 1, 3a,4,7,7a-tetrahydro-, trans- |
| CAS-85-43-8 |
| cis-DELTA4-Tetrahydrophthalic Anhydride |
| 1,3,6-Tetrahydrophthalic acid anhydride |
| NCGC00249188-01 |
| NCGC00259759-01 |
| AC-19634 |
| LS-57502 |
| trans-1,3,6-Tetrahydrophthalic anhydride |
| 1 2 3 6-Tetrahydrophthalic acid anhydride |
| LS-166032 |
| .delta.-(sup4)-Tetrahydrophthalic anhydride |
| FT-0623919 |
| FT-0631398 |
| FT-0675042 |
| 3a,4,7,7a-tetrahydro-1,3-isobenzofurandione |
| 4-Cyclohexene-1 2-dicarboxylic acid anhydride |
| EN300-18012 |
| 3a 4 7 7a-Tetrahydro-1 3-isobenzofurandione |
| 4-Cyclohexene-12-dicarboxylic anhydride (8CI) |
| Cyclohex-4-en-1,2-dicarboxylic acid anhydride |
| D77853 |
| 4-Cyclohexene-1 2-dicarboxylic anhydride (8CI) |
| 3a,4,7,7a-Tetrahydro-2-benzofuran-1,3-dione # |
| AB-131/40207535 |
| 1,3-isobenzofurandiona, 3a, 4,7,7 a-tetrahidro- |
| Q-201815 |
| Q26840954 |
| F0001-2250 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|