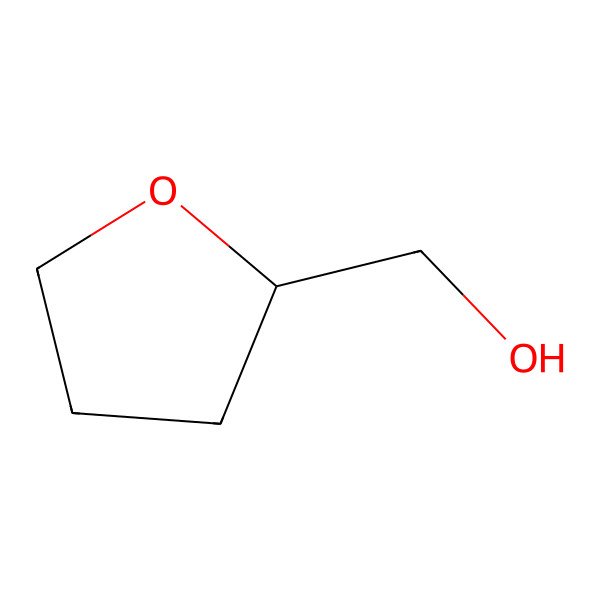
| 97-99-4 |
| (Tetrahydrofuran-2-yl)methanol |
| Tetrahydro-2-furanmethanol |
| oxolan-2-ylmethanol |
| THFA |
| 2-Furanmethanol, tetrahydro- |
| Tetrahydro-2-furanylmethanol |
| QO Thfa |
| Furfuryl alcohol, tetrahydro- |
| Tetrahydro-2-furancarbinol |
| Tetrahydrofuryl carbinol |
| Tetrahydro-2-furfuryl alcohol |
| Tetrahydro-2-furylmethanol |
| Tetrahydrofurylalkohol |
| 2-(Hydroxymethyl)tetrahydrofuran |
| tetrahydrofuran-2-ylmethanol |
| Tetrahydrofurylmethanol |
| FEMA No. 3056 |
| TETRAHYDROFURFURYLALCOHOL |
| Tetrahydrofurfurylalkohol |
| tetrahydro furfuryl alcohol |
| Oxolan-2-methanol |
| NSC 15434 |
| Tetrahydrofurylalkohol [Czech] |
| 2-Hydroxymethyl-Tetrahydrofuran |
| CCRIS 2923 |
| DTXSID1029128 |
| HSDB 5314 |
| Tetrahydrofurfurylalkohol [Czech] |
| EINECS 202-625-6 |
| MFCD00005372 |
| Furanmethanol, tetrahydro- |
| BRN 0102723 |
| UNII-XD95821VF9 |
| AI3-00104 |
| .alpha.-Tetrahydrofurfuryl alcohol |
| XD95821VF9 |
| NSC-15434 |
| EC 202-625-6 |
| DTXCID909128 |
| CAS-97-99-4 |
| 93842-55-8 |
| (R)-(-)-Tetrahydrofurfurylalcohol |
| MFCD03093085 |
| MFCD04972320 |
| [(2R)-tetrahydrofuran-2-yl]methanol |
| Thfa (van) |
| tetrahydrofuranmethanol |
| tetrahydrofuran-methanol |
| (oxolan-2-yl)methanol |
| tetrahydrofuran--methanol |
| tetrahydrofuran - methanol |
| tetrahydrofuran-2-methanol |
| WLN: T5OTJ B1Q |
| 2-tetrahydrofuranyl-methanol |
| SCHEMBL4208 |
| Qo tetrahydrofurfuryl alcohol |
| (tetrahydro-2furanyl)methanol |
| 2-hydroxymethyltetrahydrofuran |
| tetrahydro-furan-2-ylmethanol |
| 2-hydroxymethyl tetrahydrofuran |
| alpha-Tetrahydrofurfuryl alcohol |
| Tetrahydrofurfuryl alcohol, 8CI |
| CHEMBL2287521 |
| rac-tetrahydrofuran-2-ylmethanol |
| THFA,Tetrahydro-2-furancarbinol |
| (RS)-tetrahydrofuran-2-methanol |
| FEMA 3056 |
| Tetrahydrofurfuryl alcohol, 98% |
| Tetrahydrofurfuryl alcohol, 99% |
| (tetrahydro-furan-2-yl)-methanol |
| CHEBI:137944 |
| rac-2-hydroxymethyl-tetrahydrofuran |
| NSC15434 |
| Tetrahydrofurfuryl alcohol, >=98% |
| Tox21_201324 |
| Tox21_303393 |
| STL280495 |
| AKOS000118902 |
| AKOS016352940 |
| AM81815 |
| CS-W004058 |
| LS-3115 |
| SB44757 |
| SB44818 |
| TETRAHYDROFURFURYL ALCOHOL [MI] |
| TETRAHYDROFURFURYL ALCOHOL [FCC] |
| NCGC00249026-01 |
| NCGC00257256-01 |
| NCGC00258876-01 |
| TETRAHYDROFURFURYL ALCOHOL [FHFI] |
| TETRAHYDROFURFURYL ALCOHOL [HSDB] |
| TETRAHYDROFURFURYL ALCOHOL [INCI] |
| SY106537 |
| SY227666 |
| FT-0605078 |
| FT-0650427 |
| FT-0695328 |
| FT-0771179 |
| T0106 |
| TETRAHYDROFURFURYL ALCOHOL, (+/-)- |
| EN300-19349 |
| Tetrahydrofurfuryl alcohol, analytical standard |
| D77646 |
| A845782 |
| J-524856 |
| Q2406741 |
| F0001-0790 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|