| 112-60-7 |
| 2,2'-((Oxybis(ethane-2,1-diyl))bis(oxy))diethanol |
| HI-Dry |
| 3,6,9-Trioxaundecane-1,11-diol |
| Carbitol, diethyl |
| Ethanol, 2,2'-[oxybis(2,1-ethanediyloxy)]bis- |
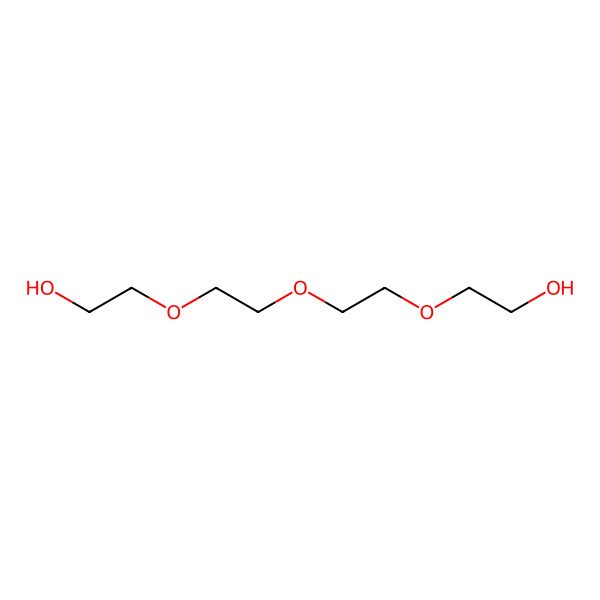
| Tetra(ethylene glycol) |
| 2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethanol |
| 3,6,9-Trioxaundecan-1,11-diol |
| NSC 1262 |
| PEG-4 |
| MFCD00002879 |
| Bis[2-(2-hydroxyethoxy)ethyl] ether |
| HSDB 843 |
| Ethanol, 2,2'-(oxybis(ethyleneoxy))di- |
| PROTAC Linker 18 |
| C8H18O5 |
| 2,2'-[oxybis(2,1-ethanediyloxy)]diethanol |
| 2,2'-[oxybis(ethane-2,1-diyloxy)]diethanol |
| EINECS 203-989-9 |
| Tetraethylene glycol, 99% |
| BRN 1634320 |
| AI3-01838 |
| 2,2'-((oxybis(ethane-2,1-diyl))bis(oxy))bis(ethan-1-ol) |
| DTXSID9026922 |
| CHEBI:44920 |
| 2-(2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy)ethanol |
| Ethanol, 2,2'-(oxybis(2,1-ethanediyloxy))bis- |
| 2-{2-[2-(2-hydroxyethoxy)ethoxy]ethoxy}ethan-1-ol |
| 127821-00-5 |
| 2,2'-[oxybis(2,1-ethanediyloxy)]bisethanol |
| EC 203-989-9 |
| 125481-05-2 |
| 157299-02-0 |
| 9004-76-6 |
| DTXCID806922 |
| CAS-112-60-7 |
| PG4 |
| HO-PEG4-OH |
| tetraethylenglycol |
| Tetraethyleneglycol |
| tetra-ethylene glycol |
| TTG (CHRIS Code) |
| OH-PEG4-OH |
| D0D9PI |
| SCHEMBL15101 |
| WLN: Q2O2O2O2Q |
| UNII-R95B8J264J |
| CHEMBL1235254 |
| 3,9-Trioxaundecane-1,11-diol |
| NSC1262 |
| R95B8J264J |
| Tetraethylene Glycol Reagent Grade |
| NSC-1262 |
| PREDNISOLONETEBUTATE(200MG) |
| Tox21_201629 |
| Tox21_303245 |
| STL268854 |
| 2,2'-(Oxybis(ethyleneoxy))diethanol |
| Ethanol,2'-[oxybis(ethyleneoxy)]di- |
| AKOS000119933 |
| CS-W019531 |
| HY-W018745 |
| 1,11-Dihydroxy-3,6,9-trioxaundecane |
| NCGC00164038-01 |
| NCGC00164038-02 |
| NCGC00257035-01 |
| NCGC00259178-01 |
| BP-20324 |
| DS-17878 |
| SY010865 |
| LS-148938 |
| FT-0645137 |
| FT-0689175 |
| FT-0763791 |
| T0099 |
| EN300-19499 |
| Ethanol,2'-[oxybis(2,1-ethanediyloxy)]bis- |
| UNDECANE-1,11-DIOL, 3,6,9-TRIOXA- |
| W18472 |
| 2-{2-[2-(2-Hydroxy-ethoxy)-ethoxy]-ethoxy}- |
| 2-(2-(2-(2-hydroxy-ethoxy)ethoxy)ethoxy)ethanol |
| J-519899 |
| 2-{2-[2-(2-hydroxy-ethoxy)-ethoxy]-ethoxy}-ethanol |
| Q12430182 |
| ETHANOL,2,2'-[OXYBIS(2,1-ETHANEDIYLOXY)]BIS- |
| F8889-3081 |
| 2,2'-(2,2'-oxybis(ethane-2,1-diyl)bis(oxy))diethanol |
|
There are more than 10 synonyms. If you wish to see them all click here.
|