| 315-37-7 |
| Delatestryl |
| Testosterone heptanoate |
| Androtardyl |
| Testosterone enantate |
| Testosterone heptylate |
| Atlatest |
| Testanthate |
| Testinon |
| Testoenant |
| Testostroval |
| Everone |
| Orquisteron-E |
| Exten test |
| Depo-Testro Med |
| Testosterone oenanthate |
| Andropository |
| Durathate |
| Testenate |
| Testosterone heptoate |
| Reposo-TMD |
| Primotestone |
| Malogen L.A. |
| DePatestrye |
| Testosterone 17-enanthate |
| NSC-17591 |
| Malogen L.A.200 |
| Reposo TMD |
| Testonenant |
| Ditate |
| Andro L.A. 200 |
| 17-Hydroxyandrost-4-en-3-one, 17-heptanoate |
| Delatest |
| Testate |
| Xyosted |
| Testosterone, heptanoate |
| Heptanoic acid, ester with testosterone |
| 17-((1-Oxoheptyl)oxy)androst-4-en-3-one |
| 17beta-Enanthoxyandrost-4-en-3-one |
| 4-Androsten-3-one 17beta-enanthate |
| Androgyn L.A. |
| Androst-4-en-3-one, 17-[(1-oxoheptyl)oxy]-, (17b)- |
| Testosterone ethanate |
| CCRIS 7082 |
| DEA No. 4000 |
| 17beta-Hydroxyandrost-4-en-3-one enanthate |
| EINECS 206-253-5 |
| Androst-4-en-3-one, 17-((1-oxoheptyl)oxy)-, (17beta)- |
| Testosterone enanthate ciii |
| BRN 3170544 |
| CHEBI:9464 |
| UNII-7Z6522T8N9 |
| Testosterone enanthate [USP:JAN] |
| Testosterone enanthate [USAN:JAN] |
| Androst-4-en-3-one, 17beta-hydroxy-, heptanoate |
| C26H40O3 |
| 7Z6522T8N9 |
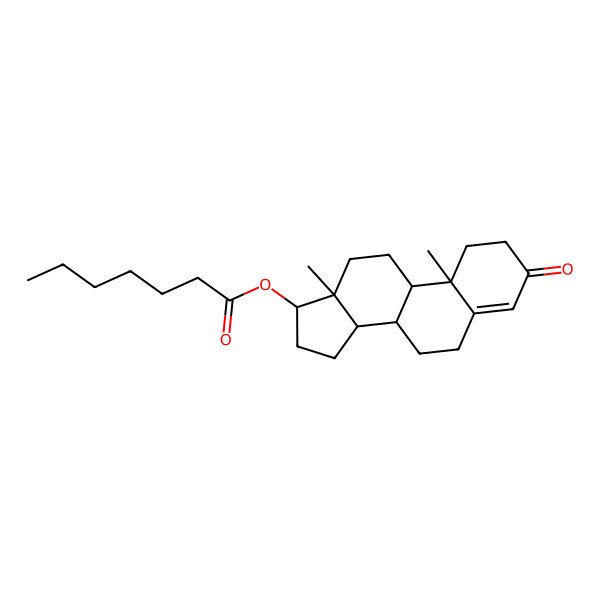
| [(8R,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl] heptanoate |
| Androst-4-en-3-one, 17-(1-oxoheptyl)oxy-, (17beta)- |
| 17-heptanoyl-17beta-hydroxyandrost-4-en-3-one |
| 4-08-00-00979 (Beilstein Handbook Reference) |
| Theramex |
| Androst-4-en-3-one, 17-[(1-oxoheptyl)oxy]-, (17.beta.)- |
| 17-[(1-Oxoheptyl)oxy]androst-4-en-3-one |
| Androgyn L.A |
| Malogen L.A |
| Delatestryl (TN) |
| Testosteroni enantas |
| Ditate (Salt/Mix) |
| Deladumone (Salt/Mix) |
| Testosterone heptoic acid |
| Enanthic acid testosterone |
| SCHEMBL42687 |
| Androgyn L.A. (Salt/Mix) |
| Testosterone 17beta-heptanoate |
| CHEMBL1200335 |
| Androst-4-en-3-one, heptanoate |
| DTXSID701016540 |
| Testosterone 17beta-heptanoic acid |
| TESTOSTERONE ENANTHATE [MI] |
| DRG-0260 |
| NSC17591 |
| Testosterone enanthate (JP17/USP) |
| TESTOSTERONE ENANTHATE [JAN] |
| LMST02020075 |
| s3717 |
| Androst-4-en-3-one,(17.beta.)- |
| TESTOSTERONE ENANTATE [MART.] |
| TESTOSTERONE ENANTHATE [VANDF] |
| AKOS015960945 |
| TESTOSTERONE ENANTATE [WHO-IP] |
| CCG-268655 |
| CS-O-05333 |
| DB13944 |
| TESTOSTERONE ENANTHATE [USP-RS] |
| TESTOSTERONE ENANTHATE [WHO-DD] |
| 4-Androsten-3-one 17.beta.-enanthate |
| 3-oxoandrost-4-en-17beta-yl heptanoate |
| (8R,9S,10R,13S,14S,17S)-10,13-Dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl heptanoate |
| AC-12599 |
| DS-11585 |
| Testosterone enanthate, analytical standard |
| 4-Androsten-17beta-ol-3-one 17-enanthate |
| LS-148818 |
| TESTOSTERONE ENANTATE [EP MONOGRAPH] |
| TESTOSTERONE ENANTHATE [ORANGE BOOK] |
| TESTOSTERONI ENANTAS [WHO-IP LATIN] |
| 17beta-hydroxyandrost-4-en-3-one heptanoate |
| TESTOSTERONE ENANTHATE CIII [USP-RS] |
| TESTOSTERONE ENANTHATE [USP MONOGRAPH] |
| WLN: L E5 B666 OV MUTJ A E FOV6 |
| C08157 |
| D00958 |
| DITATE-DS COMPONENT TESTOSTERONE ENANTHATE |
| 17-Hydroxyandrost-4-en-3-one, 17-heptanoic acid |
| SR-01000942262 |
| SR-01000942262-1 |
| TESTOSTERONE ENANTHATE COMPONENT OF DITATE-DS |
| W-106891 |
| Androst-4-en-3-one, 17.beta.-hydroxy-, heptanoate |
| Q27108402 |
| 3-Oxoandrost-4-en-17-yl heptanoate, (17.beta.)- # |
| 17.BETA.-(HEPTANOYLOXY)ANDROST-4-EN-3-ONE [WHO-IP] |
| Androst-4-en-3-ona, 17-[(1-oxoheptil)oxi]-, (17beta )- |
| ANDROST-4-EN-3-ONE, 17-(1-OXOHEPTYL)OXY-, (17.BETA.)- |
| Testosterone enantate, European Pharmacopoeia (EP) Reference Standard |
| Testosterone enanthate, United States Pharmacopeia (USP) Reference Standard |
| Testosterone enantate for peak identification, European Pharmacopoeia (EP) Reference Standard |
| Testosterone enantate for system suitability, European Pharmacopoeia (EP) Reference Standard |
|
There are more than 10 synonyms. If you wish to see them all click here.
|