| Rickamicin |
| Sisomycin |
| 32385-11-8 |
| Sissomicin |
| Siseptin |
| Antibiotic 6640 |
| Antibiotic 66-40 |
| Sch 13475 |
| Dehydrogentamicin Cla |
| Sch-13475 |
| X55XSL74YQ |
| CHEBI:9169 |
| Sisomin |
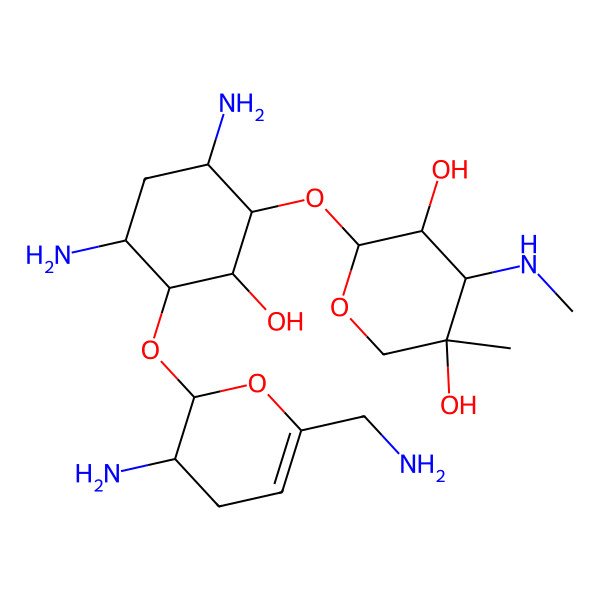
| D-Streptamine, O-3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-6)-O-(2,6-diamino-2,3,4,6-tetradeoxy-alpha-D-glycero-hex-4-enopyranosyl-(1-4))-2-deoxy- |
| Sisomicinum |
| Sisomicina |
| Sisomicine |
| (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[[(2S,3R)-3-amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yl]oxy]-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol |
| Sisomicine [INN-French] |
| Sisomicinum [INN-Latin] |
| Salvamina |
| Sisomicina [INN-Spanish] |
| Siseptin sulfate |
| (1S,2S,3R,4S,6R)-4,6-diamino-3-[(2S,3R)-3-amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yloxy]-2-hydroxycyclohexyl 3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranoside |
| (1s,2s,3r,4s,6r)-4,6-Diamino-3-{[(2s,3r)-3-Amino-6-(Aminomethyl)-3,4-Dihydro-2h-Pyran-2-Yl]oxy}-2-Hydroxycyclohexyl 3-Deoxy-4-C-Methyl-3-(Methylamino)-Beta-L-Arabinopyranoside |
| (2R,3R,4R,5R)-2-(((1S,2S,3R,4S,6R)-4,6-diamino-3-(((2S,3R)-3-amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)-5-methyl-4-(methylamino)tetrahydro-2H-pyran-3,5-diol |
| O-2,6-Diamino-2,3,4,6-tetradeoxy-alpha-D-glycero-hex-4-enopyranosyl-(1-4)-O-(3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-6))-2-deoxy-D-streptamine |
| EINECS 251-018-2 |
| UNII-X55XSL74YQ |
| BRN 1357913 |
| Sisomicin [USAN:INN:BAN] |
| Sch13475 |
| sisomicin-sulfate |
| SiS |
| SISO |
| SISOMICIN [INN] |
| SISOMICIN [MI] |
| Sisomicin (USAN/INN) |
| SISOMICIN [USAN] |
| SISOMICIN [WHO-DD] |
| SCHEMBL49395 |
| O-2,6-Diamino-2,3,4,6-tetradeoxy-alpha-D-glycero-hex-4-enopyranosyl-(1-4)-O-(3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-6)-2-deoxy-D-streptamine |
| O-3-Deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl-(1-4)-O-(2,6-diamino-2,3,4,6-tetradeoxy-alpha-D-glycero-hex-4-enopyranosyl-(1-6))-2-deoxy-L-streptamine |
| CHEMBL221886 |
| GTPL10858 |
| HY-B1222A |
| URWAJWIAIPFPJE-YFMIWBNJSA-N |
| (2S-CIS)-4-O-(3-AMINO-6-(AMINOMETHYL)-3,4-DIHYDRO-2H-PYRAN-2-YL)-2-DEOXY-6-O-(3-DEOXY-4-C-METHYL-3-(METHYLAMINO)-.BETA.-L-ARABINOPYRANOSYL)-D-STREPTAMINE |
| AKOS015895179 |
| DB12604 |
| (2S-cis)-4-O-(3-Amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yl)-2-deoxy-6-O-(3-deoxy-4-C-methyl-3-(methylamino)-beta-L-arabinopyranosyl)-D-streptamine |
| CS-0013807 |
| C00494 |
| D02544 |
| GENTAMICIN SULFATE IMPURITY A [EP IMPURITY] |
| NETILMICIN SULFATE IMPURITY A [EP IMPURITY] |
| Q3962119 |
| (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[[(2S,3R)-3-amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yl]oxy]-2-hydroxy-cyclohexoxy]-5-methyl-4-(methylamino)tetrahydropyran-3,5-diol |
| (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[[3-amino-6-(aminomethyl)-3,4-dihydro-2H-pyran-2-yl]oxy]-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol |
| 2-DEOXY-4-O-(3-DEOXY-4-C-METHYL-3-(METHYLAMINO)-.BETA.-L-ARABINOPYRANOSYL)-6-O-(2,6-DIAMINO-2,3,4,6-TETRADEOXY-.ALPHA.-D-GLYCERO-HEX-4-ENOPYRANOSYL)-L-STREPTAMINE |
| O-3-DEOXY-4-C-METHYL-3-(METHYLAMINO)-.BETA-L-ARABINOPYRANOSYL-(1->4)-O-(2,6-DIAMINO-2,3,4,6-TETRADEOXY-.ALPHA.-D-GLYCERO-HEX-4-ENOPYRANOSYL-(1->6))-2-DEOXY-L-STREPTAMINE |
|
There are more than 10 synonyms. If you wish to see them all click here.
|