Silymarin
| Internal ID | 9b37516c-ed16-4237-8506-910c550b06db |
| Taxonomy | Lignans, neolignans and related compounds > Flavonolignans |
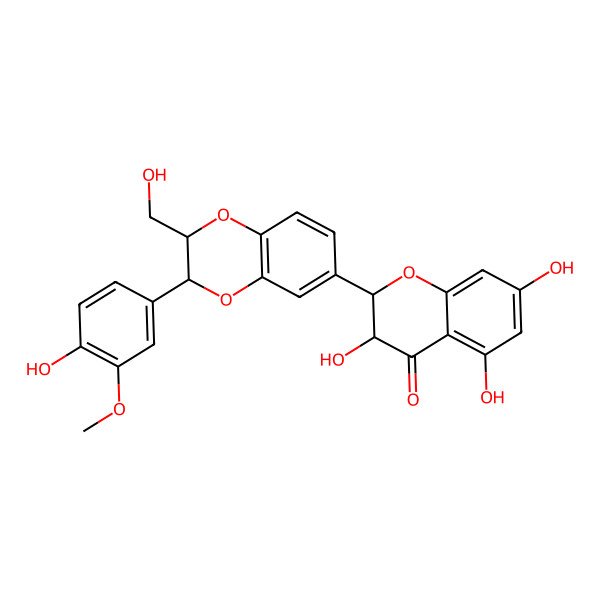
| IUPAC Name | 3,5,7-trihydroxy-2-[3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one |
| SMILES (Canonical) | COC1=C(C=CC(=C1)C2C(OC3=C(O2)C=C(C=C3)C4C(C(=O)C5=C(C=C(C=C5O4)O)O)O)CO)O |
| SMILES (Isomeric) | COC1=C(C=CC(=C1)C2C(OC3=C(O2)C=C(C=C3)C4C(C(=O)C5=C(C=C(C=C5O4)O)O)O)CO)O |
| InChI | InChI=1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3 |
| InChI Key | SEBFKMXJBCUCAI-UHFFFAOYSA-N |
| Popularity | 2,717 references in papers |
| Molecular Formula | C25H22O10 |
| Molecular Weight | 482.40 g/mol |
| Exact Mass | 482.12129689 g/mol |
| Topological Polar Surface Area (TPSA) | 155.00 Ų |
| XlogP | 2.40 |
| Silybin |
| 65666-07-1 |
| Legalon |
| Silimarin |
| Flavobion |
| Silibin |
| (2R,3R)-3,5,7-trihydroxy-2-[(2R)-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one |
| 142796-20-1 |
| Legalon 70 |
| 802918-57-6 |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| CHEMBL1293255 | P15428 | 15-hydroxyprostaglandin dehydrogenase [NAD+] |
15848.9 nM |
Potency |
via CMAUP
|
| CHEMBL3577 | P00352 | Aldehyde dehydrogenase 1A1 |
14125.4 nM |
Potency |
via CMAUP
|
| CHEMBL2903 | P16050 | Arachidonate 15-lipoxygenase |
25118.9 nM 39810.7 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL261 | P00915 | Carbonic anhydrase I |
1490 nM |
Ki |
PMID: 20674354
|
| CHEMBL205 | P00918 | Carbonic anhydrase II |
2510 nM |
Ki |
PMID: 20674354
|
| CHEMBL2885 | P07451 | Carbonic anhydrase III |
6430 nM |
Ki |
PMID: 20674354
|
| CHEMBL3729 | P22748 | Carbonic anhydrase IV |
8960 nM |
Ki |
PMID: 20674354
|
| CHEMBL3594 | Q16790 | Carbonic anhydrase IX |
10150 nM |
Ki |
PMID: 20674354
|
| CHEMBL4789 | P35218 | Carbonic anhydrase VA |
4080 nM |
Ki |
PMID: 20674354
|
| CHEMBL3969 | Q9Y2D0 | Carbonic anhydrase VB |
4560 nM |
Ki |
PMID: 20674354
|
| CHEMBL3025 | P23280 | Carbonic anhydrase VI |
9700 nM |
Ki |
PMID: 20674354
|
| CHEMBL2326 | P43166 | Carbonic anhydrase VII |
4710 nM |
Ki |
PMID: 20674354
|
| CHEMBL3242 | O43570 | Carbonic anhydrase XII |
9050 nM |
Ki |
PMID: 20674354
|
| CHEMBL3912 | Q8N1Q1 | Carbonic anhydrase XIII |
4820 nM |
Ki |
PMID: 20674354
|
| CHEMBL3510 | Q9ULX7 | Carbonic anhydrase XIV |
11640 nM |
Ki |
PMID: 20674354
|
| CHEMBL4096 | P04637 | Cellular tumor antigen p53 |
15848.9 nM |
Potency |
via CMAUP
|
| CHEMBL3397 | P11712 | Cytochrome P450 2C9 |
5000 nM |
Ki |
PMID: 16248836
|
| CHEMBL340 | P08684 | Cytochrome P450 3A4 |
12589.3 nM 10000 nM 10000 nM 12589.3 nM |
Potency Potency Potency Potency |
via CMAUP
via CMAUP via CMAUP via CMAUP |
| CHEMBL4159 | Q99714 | Endoplasmic reticulum-associated amyloid beta-peptide-binding protein |
31622.8 nM 31622.8 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha |
12589.3 nM 12589.3 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL2608 | P10253 | Lysosomal alpha-glucosidase |
35481.3 nM |
Potency |
via CMAUP
|
| CHEMBL1293224 | P10636 | Microtubule-associated protein tau |
22387.2 nM |
Potency |
via CMAUP
|
| CHEMBL1293235 | P02545 | Prelamin-A/C |
17.8 nM 17.8 nM |
Potency Potency |
via CMAUP
via Super-PRED |
| CHEMBL1697668 | Q9Y6L6 | Solute carrier organic anion transporter family member 1B1 |
9700 nM 6165.95 nM |
IC50 IC50 |
PMID: 23401473
PMID: 23571415 |
| CHEMBL1743121 | Q9NPD5 | Solute carrier organic anion transporter family member 1B3 |
2700 nM 4265.8 nM |
IC50 IC50 |
PMID: 23401473
PMID: 23571415 |
| CHEMBL1743124 | O94956 | Solute carrier organic anion transporter family member 2B1 |
4500 nM |
IC50 |
PMID: 23401473
|
| CHEMBL204 | P00734 | Thrombin |
25000 nM 20900 nM |
IC50 IC50 |
PMID: 24610996
PMID: 24610996 |
| CHEMBL1963 | P16473 | Thyroid stimulating hormone receptor |
2511.9 nM 2511.9 nM |
Potency Potency |
via CMAUP
via CMAUP |
| CHEMBL1075138 | Q9NUW8 | Tyrosyl-DNA phosphodiesterase 1 |
1995.3 nM |
Potency |
via CMAUP
|
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 99.12% | 91.11% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 97.60% | 96.09% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 94.51% | 95.56% |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha | 94.44% | 85.14% |
| CHEMBL1806 | P11388 | DNA topoisomerase II alpha | 91.41% | 89.00% |
| CHEMBL2581 | P07339 | Cathepsin D | 91.40% | 98.95% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 90.21% | 86.33% |
| CHEMBL4016 | P42262 | Glutamate receptor ionotropic, AMPA 2 | 89.78% | 86.92% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 89.75% | 97.09% |
| CHEMBL2635 | P51452 | Dual specificity protein phosphatase 3 | 89.09% | 94.00% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 88.72% | 94.45% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 87.76% | 99.17% |
| CHEMBL1860 | P10827 | Thyroid hormone receptor alpha | 86.59% | 99.15% |
| CHEMBL3038477 | P67870 | Casein kinase II alpha/beta | 83.70% | 99.23% |
| CHEMBL3194 | P02766 | Transthyretin | 83.19% | 90.71% |
| CHEMBL3401 | O75469 | Pregnane X receptor | 83.15% | 94.73% |
| CHEMBL2373 | P21730 | C5a anaphylatoxin chemotactic receptor | 81.48% | 92.62% |
| CHEMBL4478 | Q00975 | Voltage-gated N-type calcium channel alpha-1B subunit | 80.82% | 97.14% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| Anastatica hierochuntica |
| Lycium chinense |
| Silybum marianum |
| PubChem | 5213 |
| NPASS | NPC125039 |
| ChEMBL | CHEMBL1401508 |
| LOTUS | LTS0228416 |
| wikiData | Q27216069 |