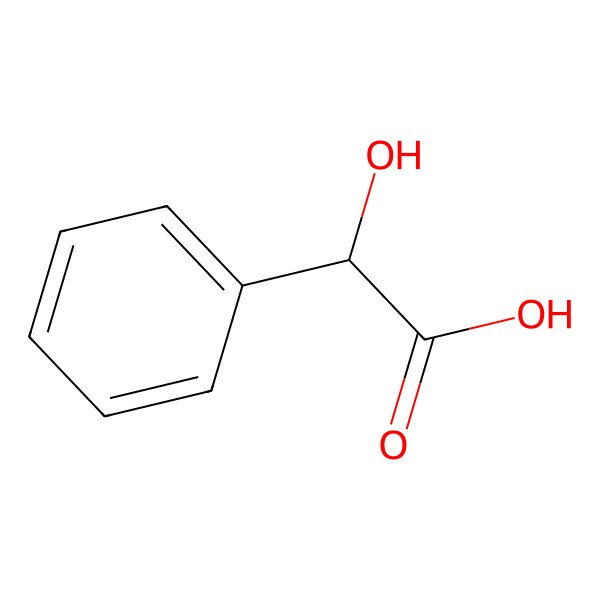
| (S)-(+)-Mandelic acid |
| (S)-Mandelic acid |
| L-mandelic acid |
| (S)-2-Hydroxy-2-phenylacetic acid |
| (2S)-2-hydroxy-2-phenylacetic acid |
| S-(+)-Mandelic acid |
| L-(+)-MANDELIC ACID |
| Mandelic acid, (S)- |
| Mandelic acid, L- |
| l(+)-mandelic acid |
| D-2-Hydroxy-2-phenylacetic acid |
| UNII-L0UMW58G3T |
| L0UMW58G3T |
| (S)-(+)-Mandelic acid, 98% e.e. |
| CHEMBL58910 |
| MLS000069517 |
| CHEBI:32800 |
| (S)-alpha-Hydroxyphenylacetic acid |
| (S)-alpha-hydroxybenzeneacetic acid |
| EINECS 241-240-8 |
| Benzeneacetic acid, .alpha.-hydroxy-, (.alpha.S)- |
| (S)-A-HYDROXYPHENYLACETIC ACID |
| alpha-Hydroxybenzeneacetic acid, (S)- |
| SMR000058582 |
| MFCD00004495 |
| Benzeneacetic acid, alpha-hydroxy-, (alphaS)- |
| EC 241-240-8 |
| (2S)-2-Hydroxy-2-phenylacetic Acid (L-Mandelic Acid) |
| DL-Amygdalic Acid |
| SMN |
| (S)-(+)-Mandelic acid, ReagentPlus(R), >=99% |
| alpha-hydroxybenzeneacetate |
| Amygdalate |
| Paramandelate |
| Phenylglycolate |
| s-mandelic acid |
| DL-Amygdalate |
| (S)-Hydroxy-phenyl-acetic acid |
| DL-Mandelate |
| 2-Phenylglycolate |
| (RS)-Mandelate |
| (S)-Mandelsaeure |
| Phenylhydroxyacetate |
| a-Hydroxy-a-toluate |
| a-Hydroxyphenylacetate |
| L-(+)mandelic acid |
| a-Hydroxybenzeneacetate |
| Benzeneacetic acid, .alpha.-hydroxy-, (S)- |
| (S)-L-Mandelic acid |
| Opera_ID_622 |
| a-Hydroxy-a-toluic acid |
| (s)(+)-mandelic acid |
| alpha-Hydroxyphenylacetate |
| a-Hydroxyphenylacetic acid |
| Mandelic acid, L-()- |
| DL-Hydroxy(phenyl)acetate |
| (+)-(s)-mandelic acid |
| a-Hydroxybenzeneacetic acid |
| 2-Phenyl-2-hydroxyacetate |
| (S)-hydroxyphenylaceticacid |
| alpha-Hydroxy-alpha-toluate |
| cid_1292 |
| (S)-(+)-Amygdalic Acid |
| 2-Hydroxy-2-phenylethanoate |
| (S)--(+)--mandelic acid |
| SCHEMBL255601 |
| cid_439616 |
| BDBM16420 |
| IWYDHOAUDWTVEP-ZETCQYMHSA-N |
| (2s)-hydroxy(phenyl)ethanoic acid |
| DTXSID301014792 |
| HMS2233J03 |
| CS-D1423 |
| STR07719 |
| AKOS006343440 |
| AKOS007930623 |
| AC-2496 |
| DB03357 |
| Benzeneacetic acid, ?-hydroxy-, (S)- |
| Benzeneacetic acid, alpha-hydroxy-, (S)- |
| AM20050159 |
| M0661 |
| (+)-.ALPHA.-HYDROXYPHENYLACETIC ACID |
| EN300-97210 |
| C01984 |
| MLS-0090889.0001 |
| L-(+)-Mandelic acid, purum, >=98.0% (T) |
| J-520416 |
| Q-201693 |
| Q27096314 |
| (S)-(+)-Mandelic acid, Vetec(TM) reagent grade, 99% |
| Z1201618616 |
| ATOMOXETINE HYDROCHLORIDE IMPURITY E [ EP IMPURITY] |
|
There are more than 10 synonyms. If you wish to see them all click here.
|